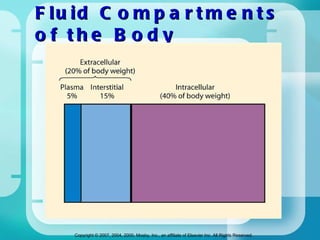
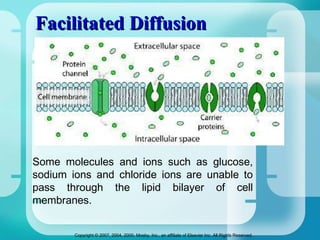
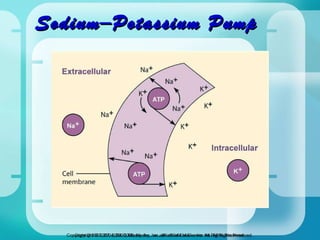
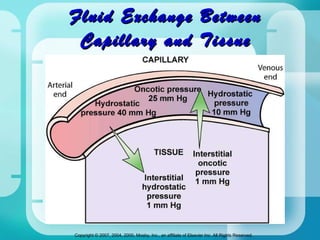
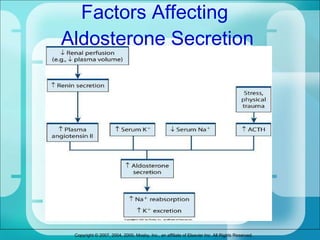
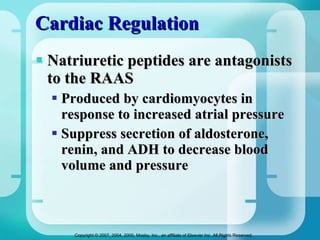
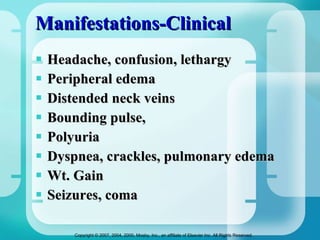
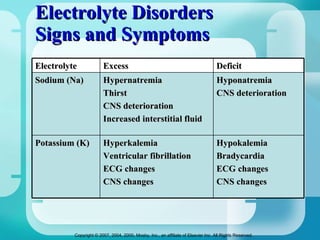
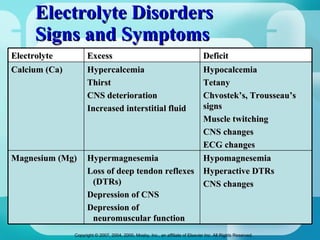
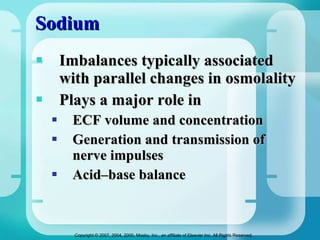
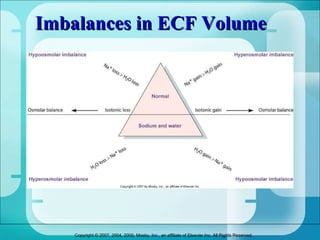
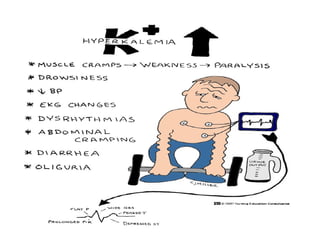
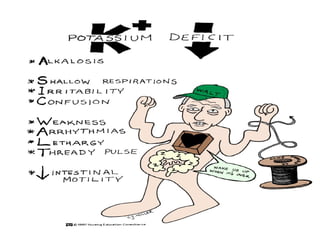
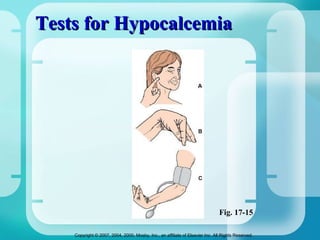
The document discusses fluid and electrolyte homeostasis in the human body. It covers the different fluid compartments, electrolytes, and mechanisms that control fluid and electrolyte movement. Common fluid and electrolyte imbalances like dehydration, edema, and electrolyte disorders are explained along with their causes, signs and symptoms, and nursing management.
![Fluid and Electrolytes Ms. Cherry Ann G. Garcia RN, MAN-IP [email_address]](https://image.slidesharecdn.com/ms-fluidelectrolytes-111025095553-phpapp02/75/Ms-fluid-electrolytes-1-2048.jpg)