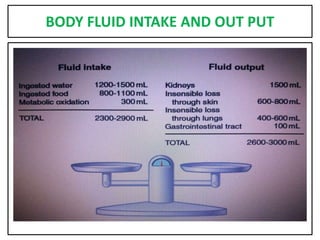
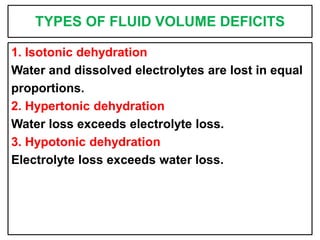
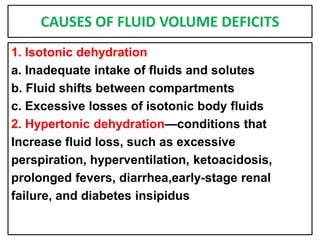
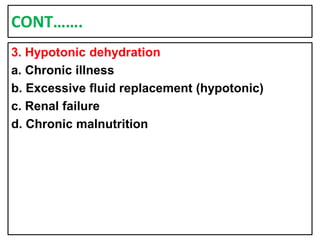
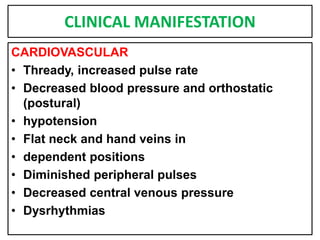
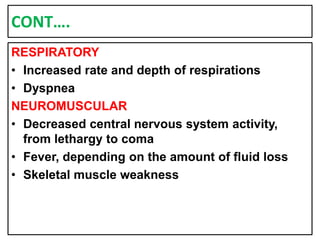
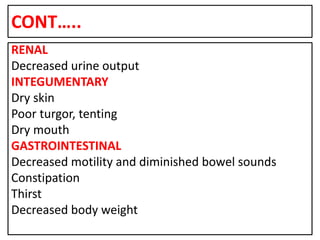
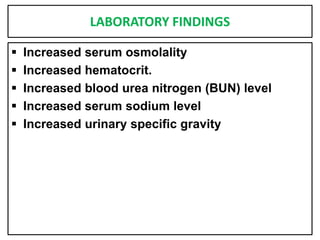
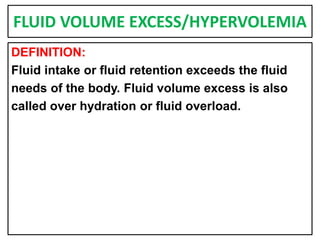
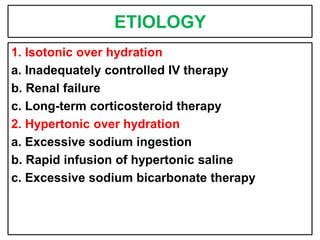
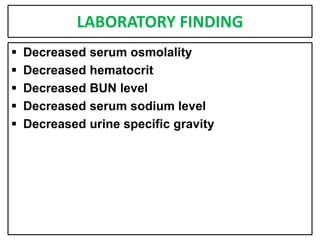
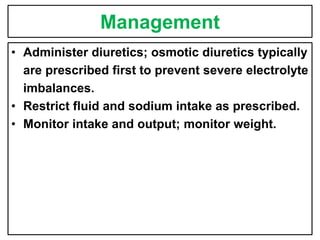
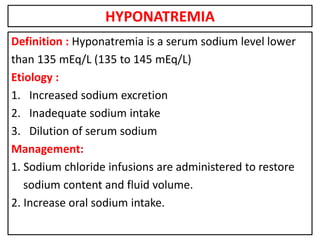
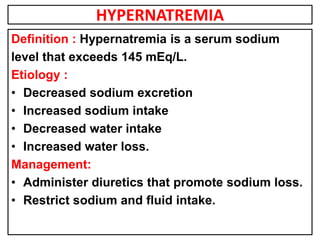
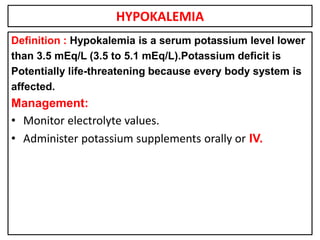
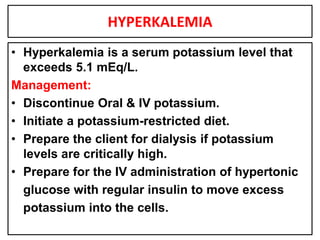
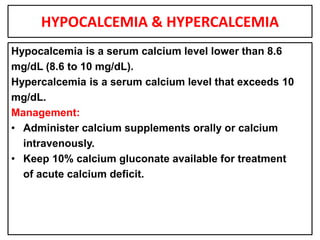
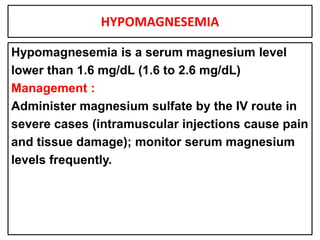
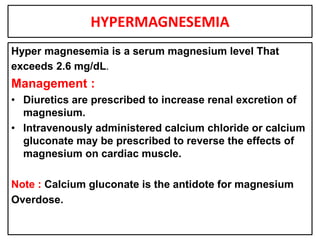
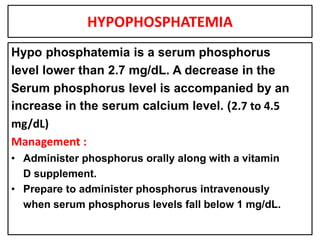
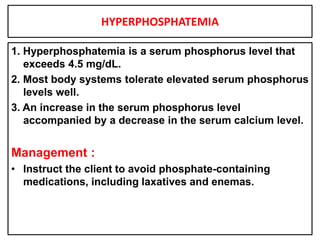
This document provides information on fluid and electrolyte balance, including definitions of key terms like electrolytes, cations, anions, and body fluid compartments. It describes the measurement of fluids and electrolytes. It explains processes of fluid transport like diffusion, osmosis, and filtration. It discusses causes and management of fluid volume deficits and excesses, including types of dehydration and fluid overload. It also covers abnormalities in specific electrolytes like sodium, potassium, calcium, magnesium, phosphorus and their management.