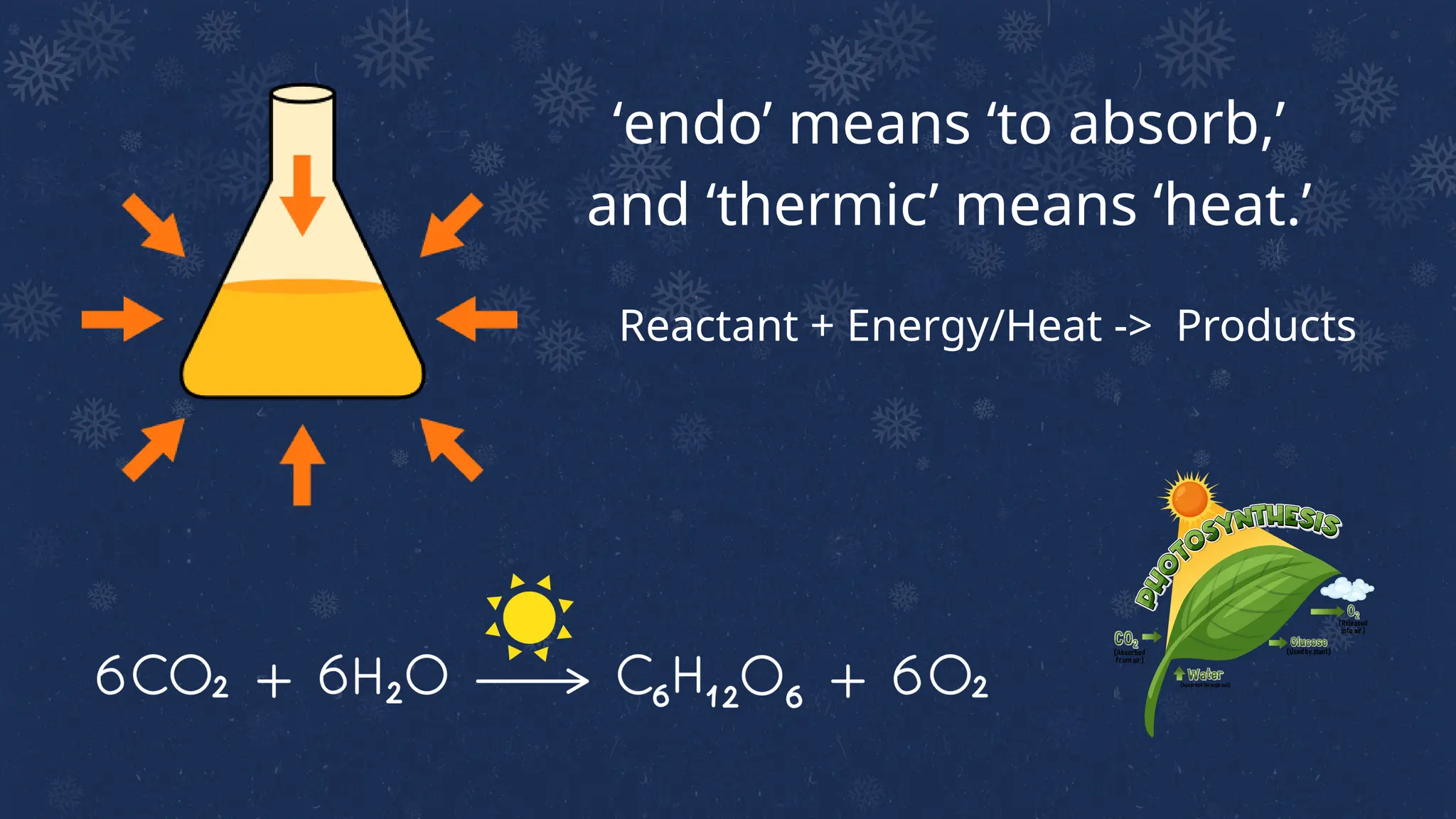
The document discusses endothermic and exothermic reactions, explaining how chemical reactions involve the breaking and forming of bonds accompanied by changes in energy. Endothermic reactions absorb energy from their surroundings, while exothermic reactions release energy, with numerous examples provided for each type. It also highlights the law of conservation of energy and the concept of activation energy required for chemical reactions.