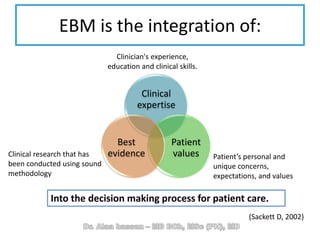
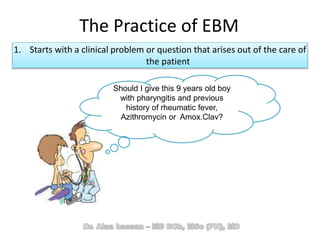
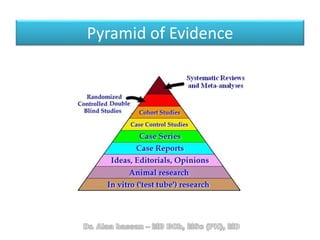
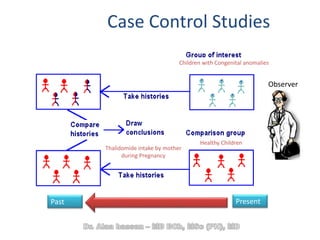
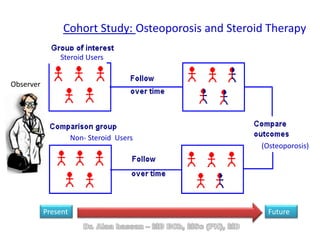
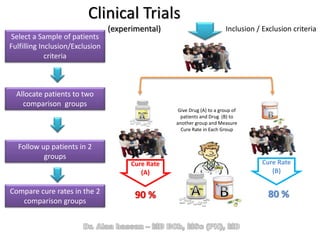
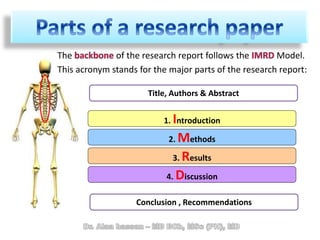
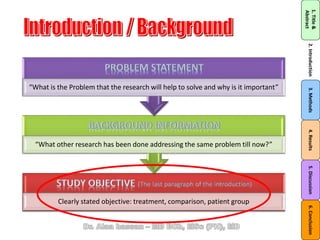
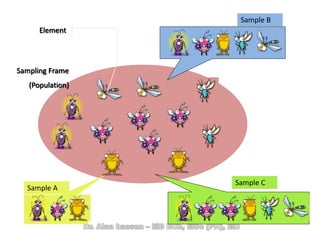
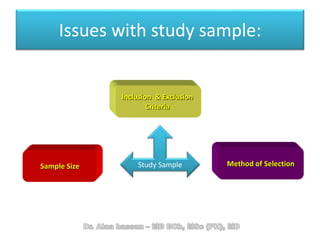
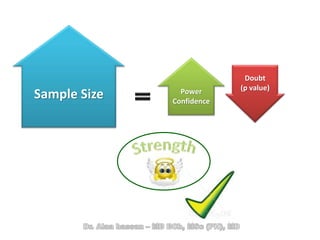
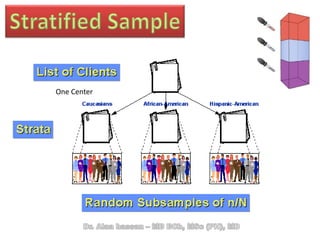
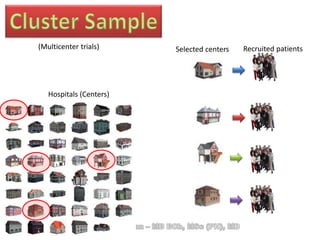
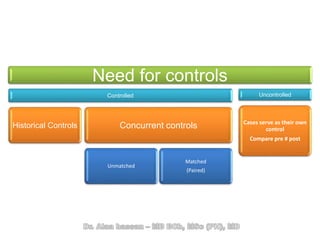
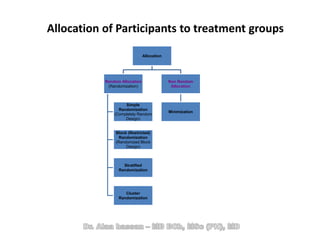
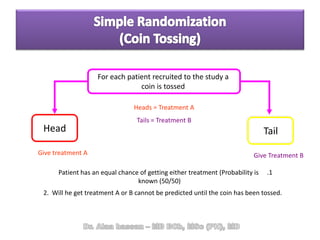
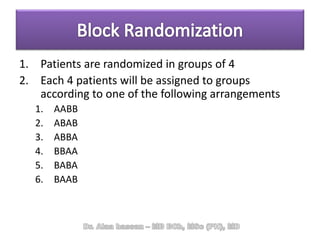
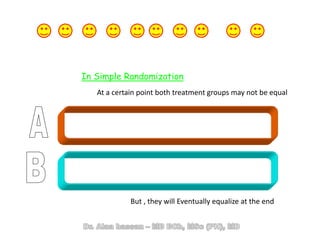
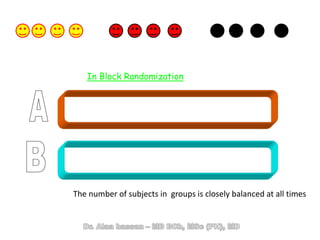
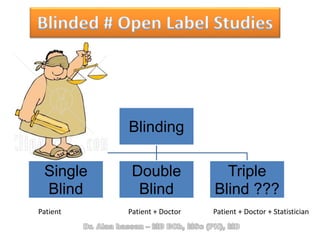
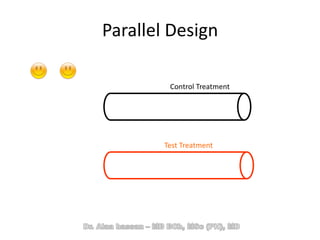
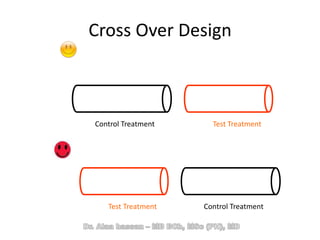
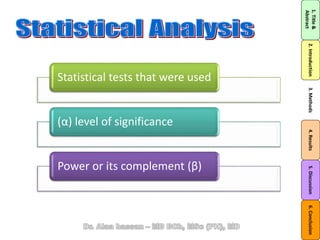
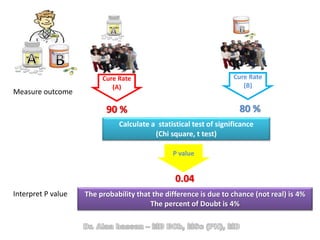
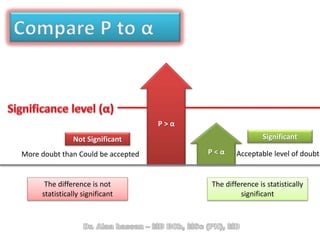
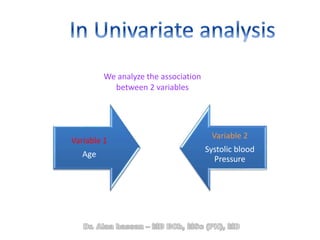
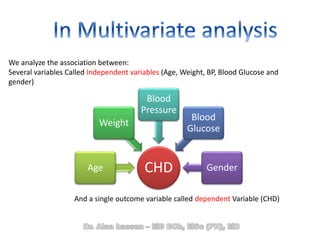
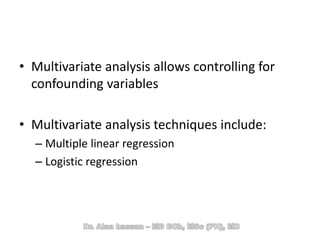
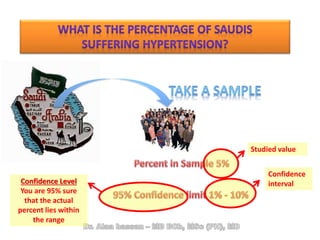
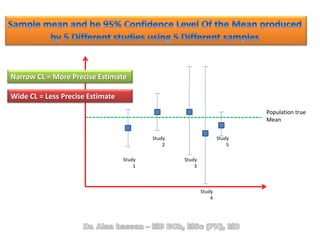
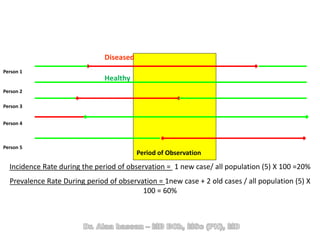
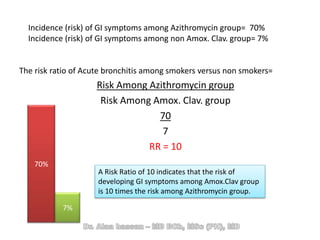
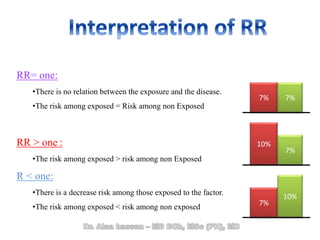
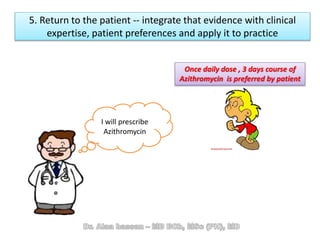
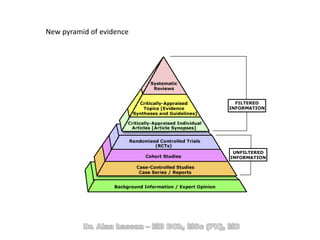
The document discusses evidence-based medicine (EBM) and summarizes its key principles. EBM involves integrating the best research evidence with a clinician's expertise and the patient's values and circumstances. It describes the 5 steps of EBM: 1) framing a clinical question based on a patient encounter, 2) finding relevant evidence, 3) critically appraising the evidence for validity and applicability, 4) applying relevant evidence to the patient, and 5) evaluating outcomes. EBM aims to formalize using literature to guide decisions by focusing on strong evidence from well-designed studies.