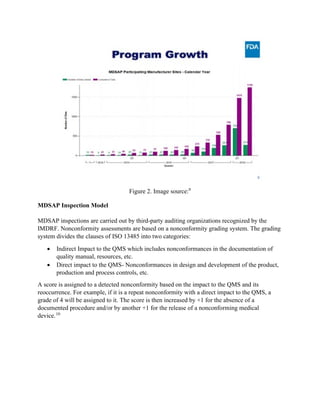
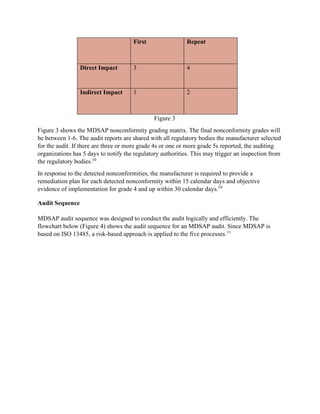
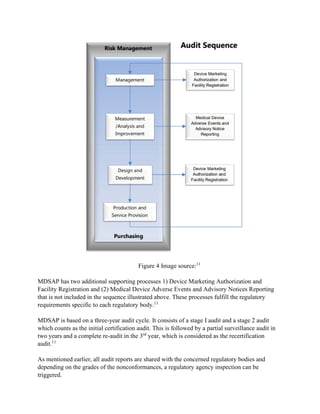
The Medical Device Single Audit Program (MDSAP), initiated in 2012 by the International Medical Device Regulators Forum, allows manufacturers to undergo a single audit that meets the regulatory requirements of multiple countries, including the U.S., Canada, Brazil, Australia, and Japan. The program has been deemed successful following a pilot from 2014 to 2016, with participating countries monitoring compliance and facilitating market access. Despite its advantages, participants may still face unannounced audits and should be prepared for stringent adherence to ISO 13485 and country-specific regulations.