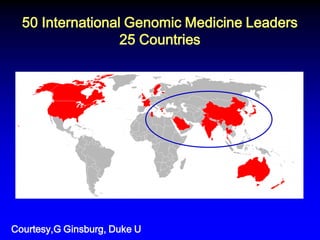
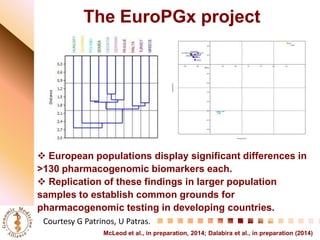
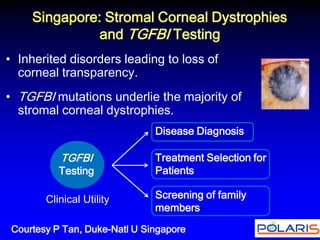
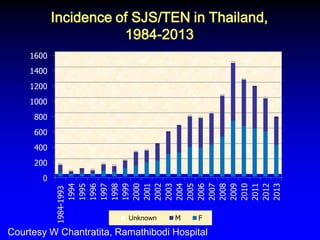
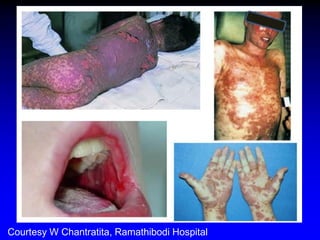
This document summarizes discussions from the 6th Genomic Medicine Colloquium hosted by the National Human Genome Research Institute. The colloquium brought together 50 international genomic medicine leaders from 25 countries to discuss opportunities for collaboration. Key areas of discussion included establishing standards for genomic data storage, implementing global pharmacogenomic screening programs, developing genomic medicine policy, and creating an international genomic medicine collaborative.