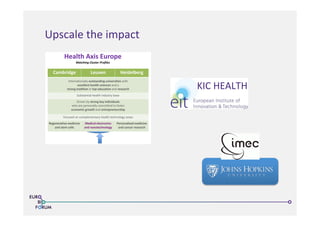
The Eurobioforum 2013 conference discussed strategies for managing disease and treatment complexities while promoting R&D innovations in healthcare. Key initiatives include the establishment of the Center for Medical Innovation (CMI) to streamline clinical applications and the Nanotechnology for Health project in Flanders, which aims to integrate academic research with industry needs. Collaborative efforts across borders and sectors are emphasized to foster advancements in health-related technologies and accelerate market introduction.