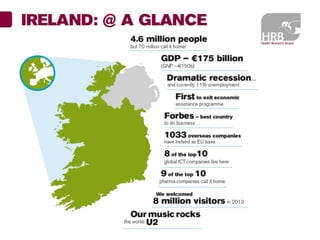
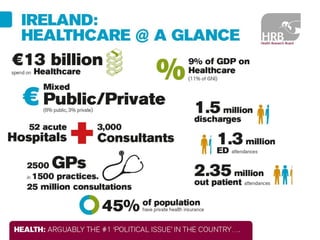
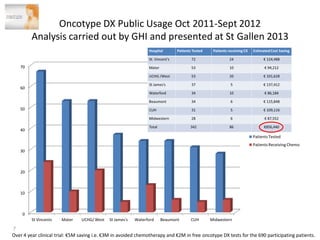
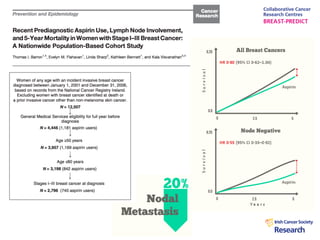
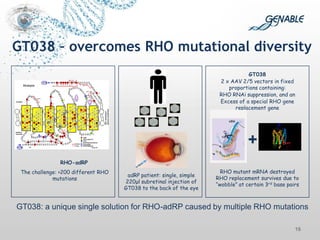
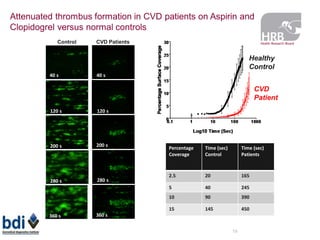
1. Graham Love discusses the development of personalized medicine in Ireland from the perspective of the Health Research Board.
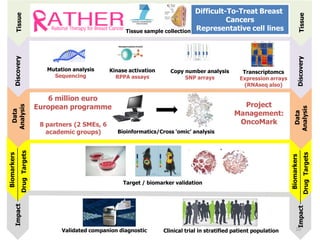
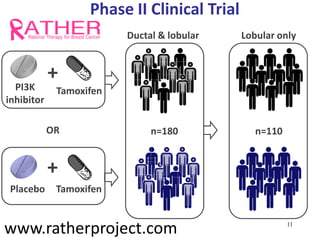
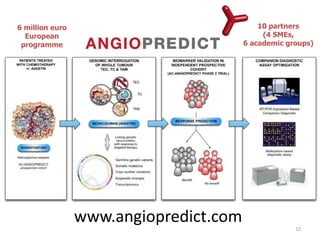
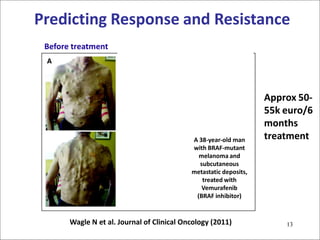
2. While some personalized cancer care exists, moving towards personalized medicine research requires large-scale sequencing efforts and clinical trials to validate biomarkers and treatments.
3. For personalized medicine to become a true revolution, there needs to be greater efforts to educate decision-makers and the general public about what personalized medicine is and its potential benefits.