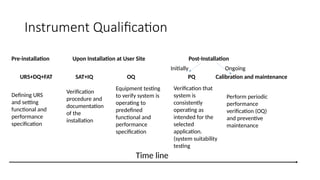
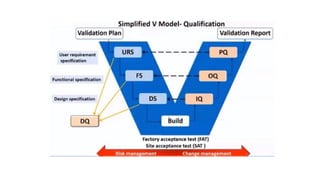
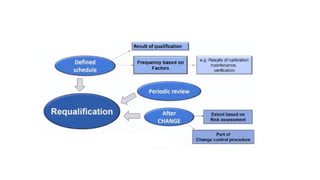
The document outlines the principles and procedures related to HPLC (High-Performance Liquid Chromatography) instrument qualification and calibration, focusing on the critical phases of validation, including design, installation, operational, and performance qualification. It emphasizes the importance of clear user requirement specifications (URS) in ensuring that instruments are properly qualified and maintained throughout their operational life. Additionally, it discusses component qualification, regulatory considerations, and the necessity for ongoing performance verification to ensure reliable analytical results.