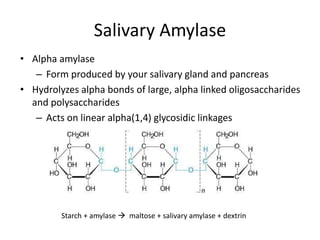
1) This experiment studied the effect of temperature and pH on the enzymatic activity of salivary amylase.
2) The optimal temperature for salivary amylase activity was found to be 37°C, as this is body temperature and the rate of reaction was highest. Activity decreased at higher and lower temperatures.
3) The optimal pH range for salivary amylase activity was pH 6.7-7, as outside this range the enzyme loses its tertiary structure and is denatured, ceasing activity.




![Introduction
Properties of Enzymes
• Large protein molecules
• Reusable (example: 2 H2O2 [catalase]→ 2 H2O + 1 O2)
• Remain unchanged
• VERY specific
• Operate at very high speeds
• Rate of reaction is dependent upon temperature,
pH, [E], and [S]
• Usually work best with 60°C
• Denaturation occurs at very high temperatures](https://image.slidesharecdn.com/enzymatic-activity-of-salivary-amylase-231020151144-9f4e7c4b/85/Enzymatic-Activity-of-Salivary-Amylase-pptx-5-320.jpg)