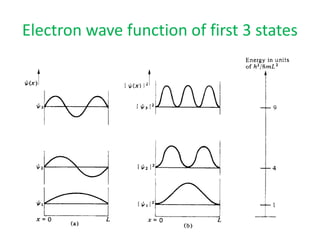
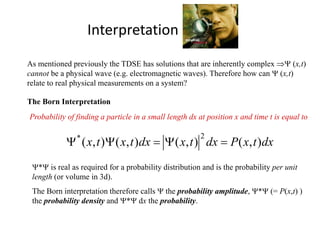
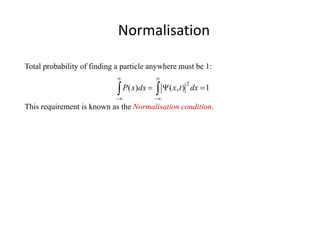
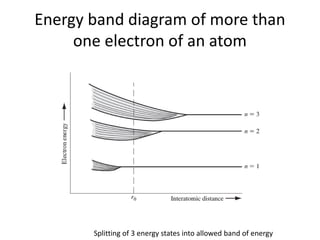
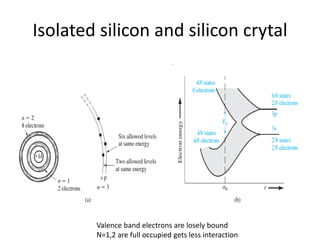
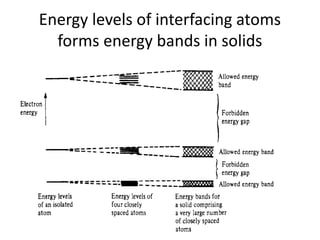
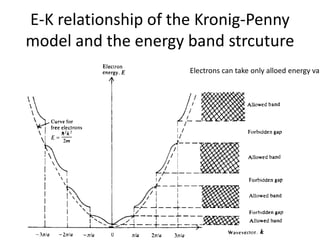
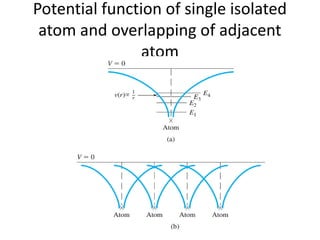
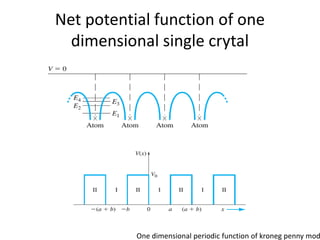
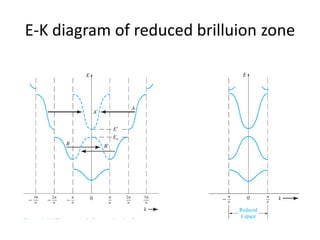
This document provides a summary of quantum mechanical concepts and solid state physics. It begins with a review of quantum mechanics and the Schrodinger equation. It then discusses the wave nature of electrons and how the Schrodinger equation describes the wavefunction and probability of finding an electron. It also covers energy band diagrams and how the periodic potential in solids leads to the formation of allowed energy bands. It discusses these concepts for isolated atoms, silicon crystals, and the one-dimensional Kronig-Penny model.