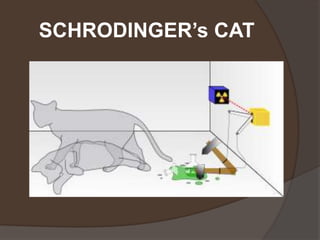
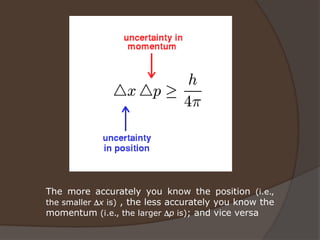
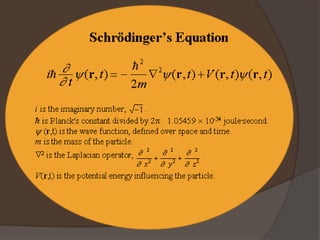The document discusses Erwin Schrödinger, an Austrian physicist known for his contributions to quantum mechanics, notably the Schrödinger equation and the concept of wave functions. It explains the implications of the Schrödinger equation in describing quantum states, introduces the thought experiment of Schrödinger's cat, and briefly covers Werner Heisenberg's uncertainty principle and its impact on measurements in quantum mechanics. Lastly, it highlights Maria Goeppert Mayer's contributions to the shell model of the atomic nucleus and nuclear pairing.