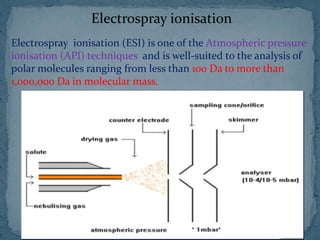
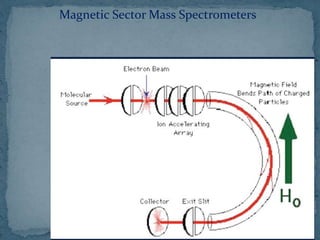
This document provides an overview of polymer analysis using mass spectrometry. It discusses what mass spectrometry is and the types of information it can provide about molecular mass and structure. It also describes how mass spectrometers work by introducing samples, ionizing them, analyzing the ions, and detecting them. Specific ionization methods like electrospray ionization and applications of mass spectrometry in areas like biotechnology and pharmaceuticals are summarized. The document concludes by outlining how mass spectrometry is used for polymer analysis by providing detailed structural and compositional information.