This document summarizes key concepts in electrostatics including:
- There are two types of electric charge - positive and negative. Opposite charges attract while like charges repel.
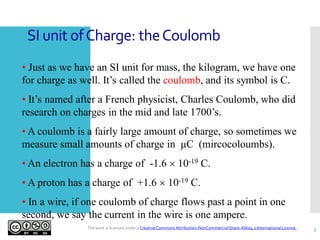
- The coulomb (C) is the SI unit for electric charge. Common charges include the -1.6x10-19 C electron and +1.6x10-19 C proton.
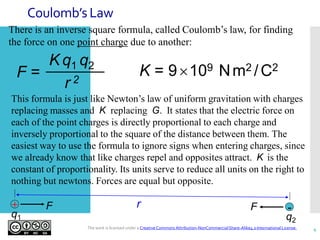
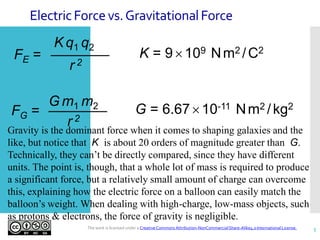
- Coulomb's law describes the inverse square relationship between the electric force between two point charges and the distance between them.