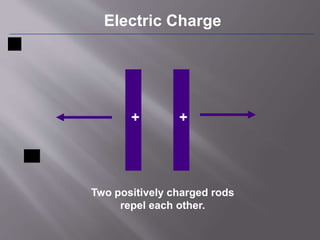
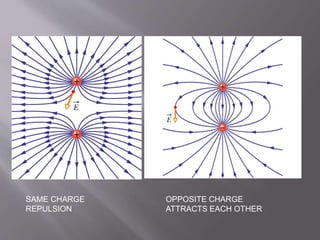
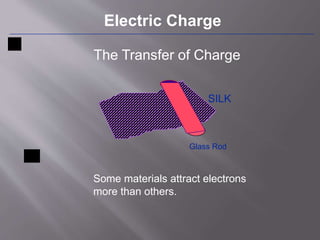
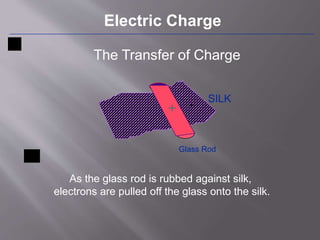
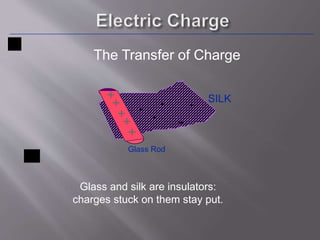
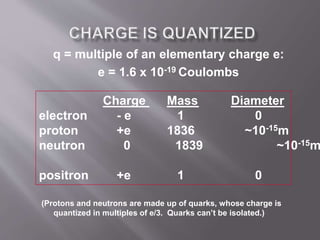
The document discusses the history and properties of electricity and electric charge. It describes how Greeks first discovered the attractive properties of amber in 600 BC and key discoveries throughout history that helped define positive and negative electric charge. The document explains how rubbing certain materials like glass and silk can cause the transfer of electrons, leaving one material with an excess of electrons and the other with a deficit. It summarizes that electric charge is a fundamental property of matter that can be either positive or negative, and like charges repel while opposite charges attract.