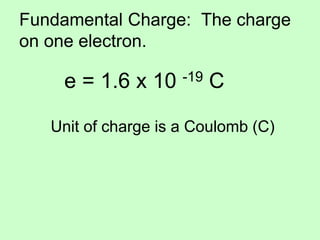
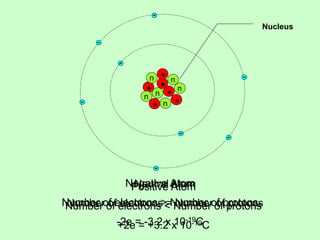
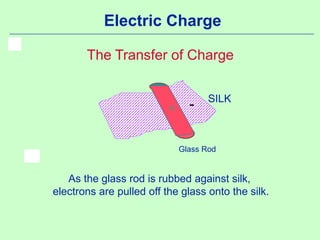
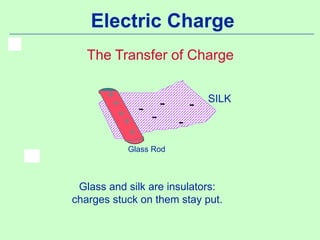
1) Electric charge is quantized and comes in multiples of the fundamental charge of an electron.
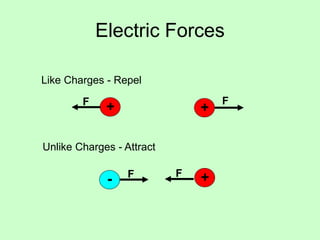
2) There are two types of charge - positive and negative - which attract or repel depending on whether they are like or unlike.
3) Coulomb's law describes the electric force between two point charges, with the force being directly proportional to the product of the charges and inversely proportional to the square of the distance between them.