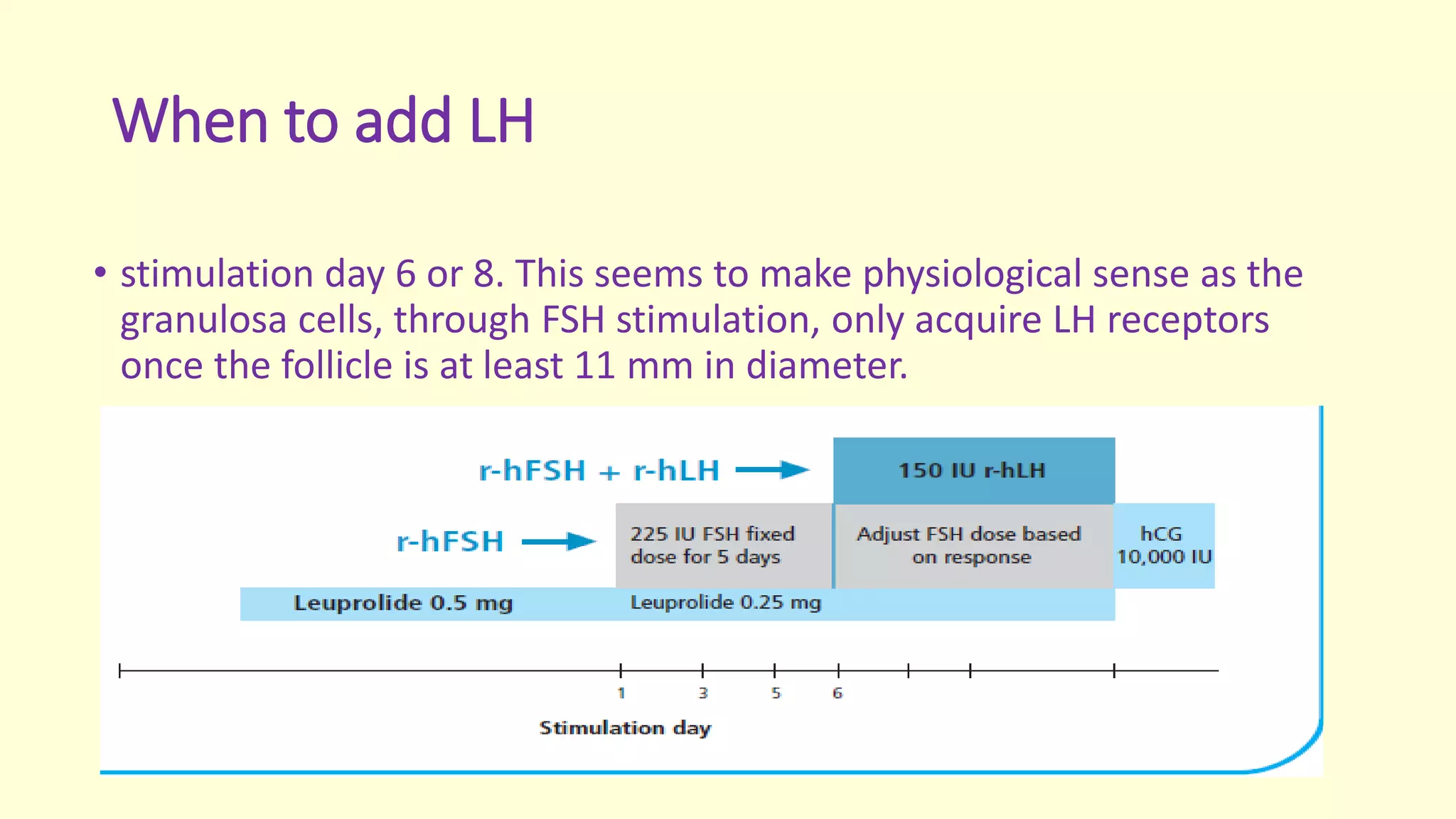
This document discusses effective protocols for superovulation when undergoing IVF treatment. It compares different ovarian stimulation protocols including long and short protocols using gonadotropin-releasing hormone (GnRH) agonists or antagonists. It also examines the use of human menopausal gonadotropin (hMG) versus recombinant follicle-stimulating hormone (r-FSH), as well as adding luteinizing hormone (LH) to stimulation. Key factors discussed include number of eggs retrieved, egg and embryo quality, risk of ovarian hyperstimulation syndrome, and pregnancy rates. The document provides guidance on optimizing protocols based on patient characteristics and treatment goals.






![Components of ovarian stimulation protocol
• The principal structure of the protocol (e.g. type, initiation and
duration of agonist or antagonist).
• The chosen gonadotrophin (e.g. r-FSH or u-FSH, with or without
additional r-h luteinizing hormone [LH]).
• The selected means of final maturation and trigger (either u-hCG or r-
hCG).
• The selected means of luteal phase support.](https://image.slidesharecdn.com/effectivesafesuperovulationnew-160603210812/75/Effective-Safe-Superovulation-7-2048.jpg)