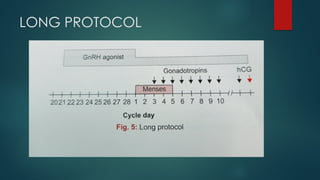
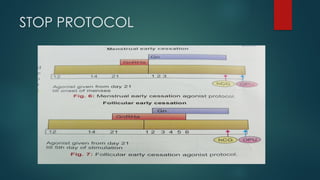
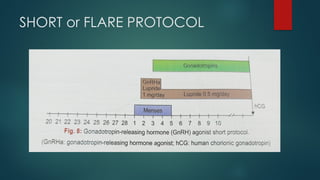
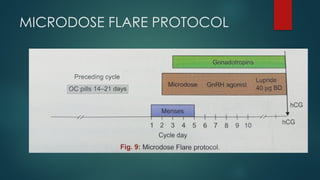
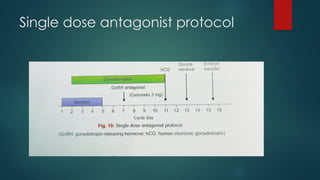
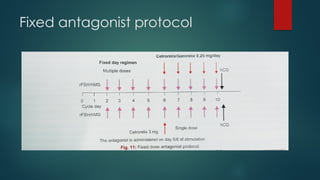
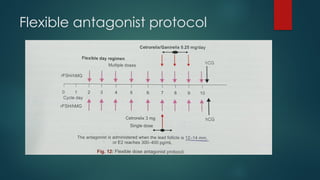
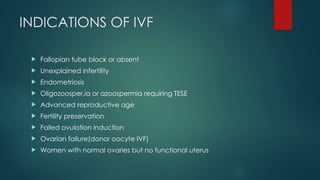
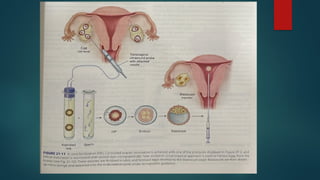
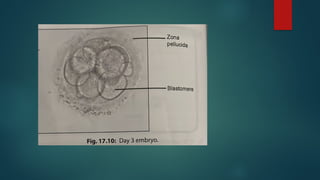
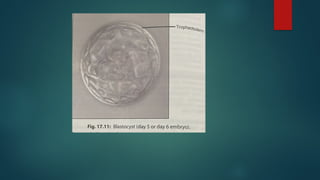
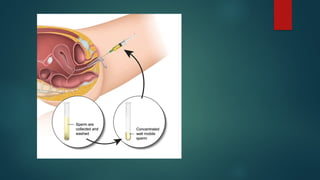
Assisted reproductive techniques (ART) are clinical and laboratory procedures used to assist infertile couples achieve pregnancy, typically involving oocyte extraction and fertilization. Various protocols exist for ovarian stimulation, including GnRH agonist and antagonist protocols, each with specific indications, advantages, and side effects. In vitro fertilization (IVF) and gamete intrafallopian transfer (GIFT) are two primary ART methods, with advancements in egg and embryo cryopreservation enhancing fertility preservation efforts.