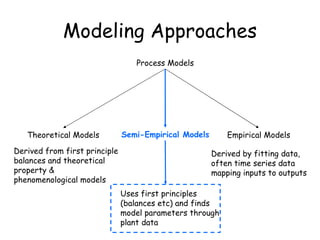
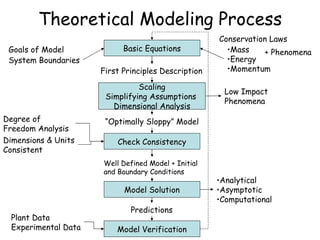
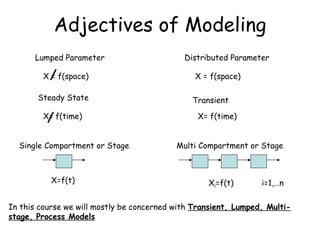
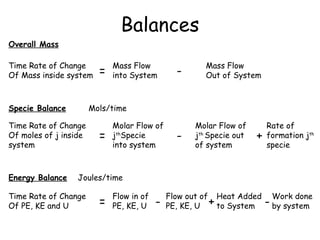
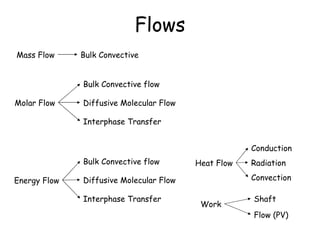
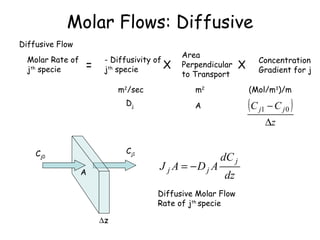
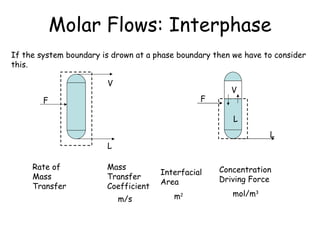
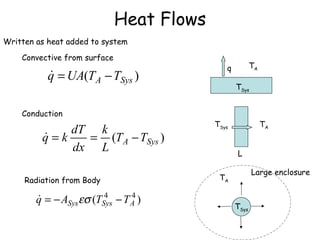
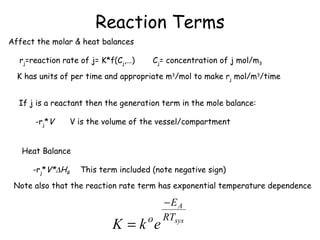
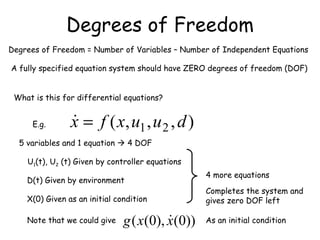
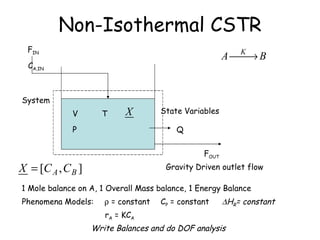
The document provides an overview of general process modeling frameworks and approaches. It discusses theoretical, empirical and semi-empirical models. Theoretical models are derived from first principles like balances and property models, empirical models fit data to map inputs to outputs, and semi-empirical models use first principles and parameter fitting. It also describes types of process models like lumped vs distributed, steady state vs transient, and single vs multi-stage models. Key modeling concepts discussed include balances, degrees of freedom analysis, and modeling flows, reactions and heat transfer.