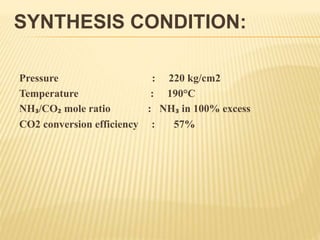
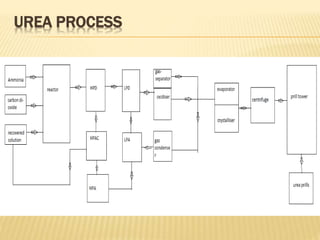
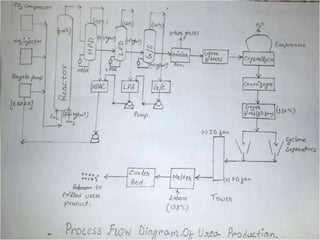
1) The document discusses the production of urea through the Mitsui Toatsu total recycle process. This involves a synthesis section where ammonia and carbon dioxide react to form ammonium carbamate.
2) In the decomposition section, heat is applied to decompose the ammonium carbamate into its components, which are then recovered.
3) The raw materials, reaction, process sections, utilities required and some common engineering problems are described at a high level.
4) Urea has various uses as a solid fertilizer and in manufacturing resins.