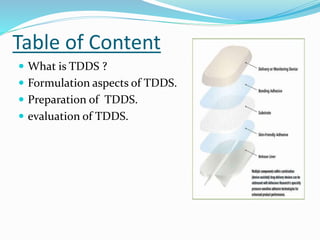
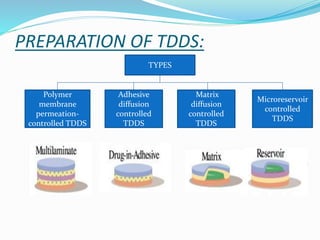
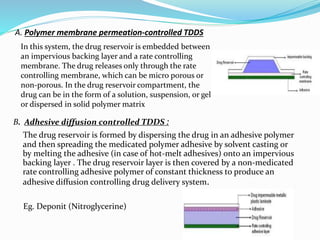
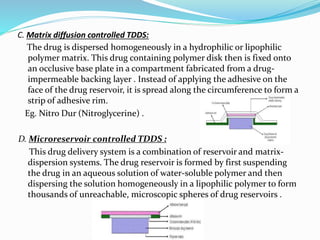
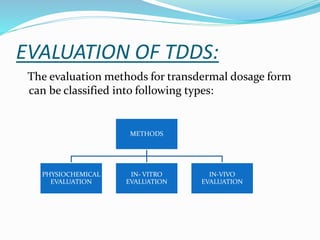
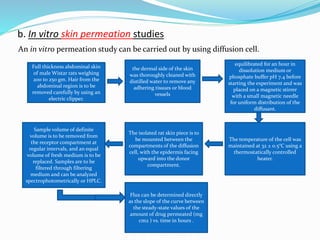
This document provides information on transdermal drug delivery systems (TDDS). It discusses the key components of TDDS formulations including the polymer matrix, drug, permeation enhancers, pressure sensitive adhesive, backing laminate and release liner. The document also describes the preparation methods for different types of TDDS and the evaluation methods used to test the physicochemical properties, in vitro drug release, and stability of TDDS. The evaluations help ensure the TDDS will safely and effectively deliver the drug through the skin as intended.