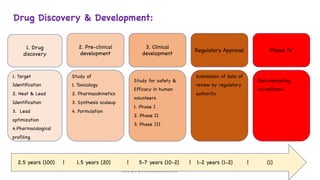
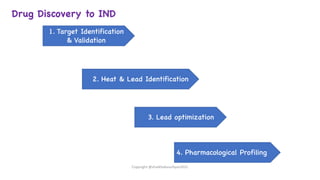
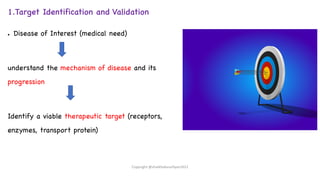
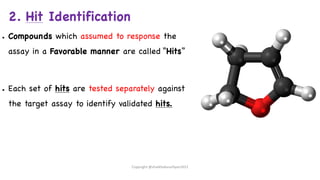
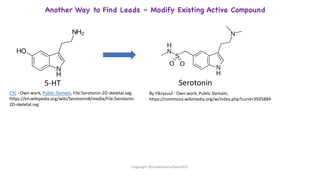
The document outlines the processes involved in drug discovery and development, including phases like target identification, hit identification, lead optimization, and clinical trials. It covers the history and ethical considerations in clinical research, emphasizing the need for informed consent and compliance with established guidelines. Additionally, it discusses the roles of ethics committees in ensuring participant safety and adherence to ethical standards during research.