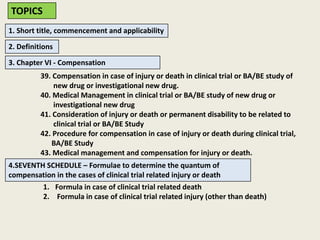
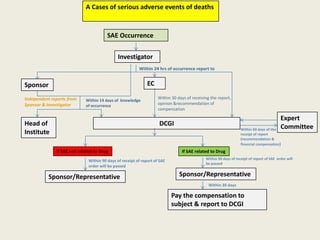
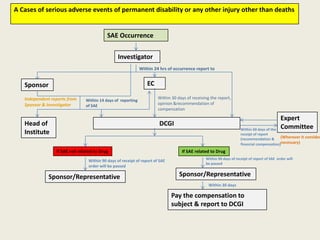
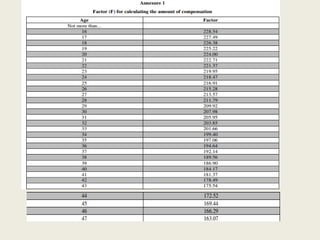
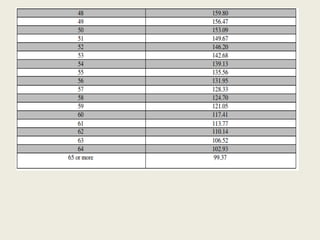
The document outlines regulations related to compensation for injury or death in clinical trials and studies involving new drugs. It stipulates the responsibilities of sponsors for medical management and financial compensation for affected subjects, detailing criteria for assessing compensation amounts based on various factors, including the severity of injury and loss of wages. The rules also establish procedures for reporting serious adverse events and calculating compensation based on defined formulas.