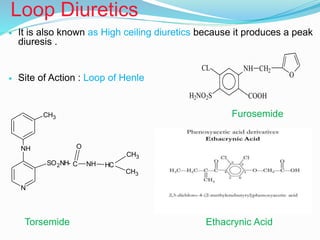
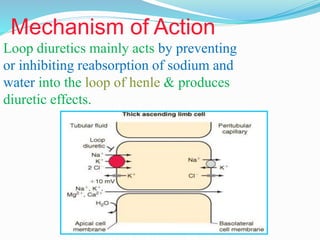
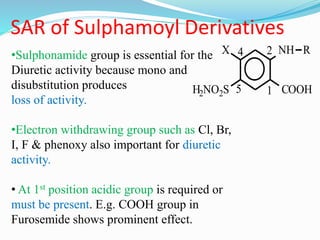
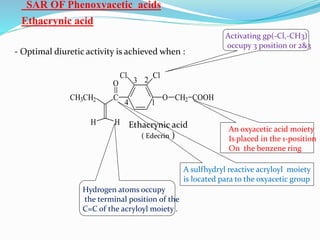
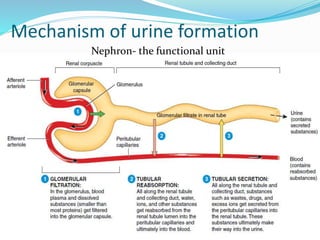
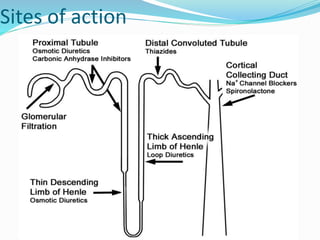
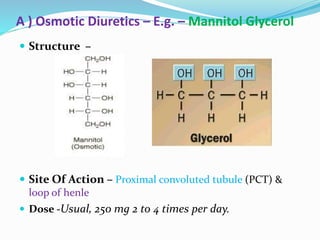
The document covers the mechanism of urine formation and provides a comprehensive overview of diuretics, including their definitions, classifications, mechanisms of action, dosages, and adverse effects. It distinguishes between mercurial and non-mercurial diuretics and details various types such as osmotic, carbonic anhydrase inhibitors, thiazides, potassium-sparing, and loop diuretics, along with their specific uses and side effects. Additionally, it emphasizes the pharmacological applications and structural requirements necessary for diuretic efficacy.





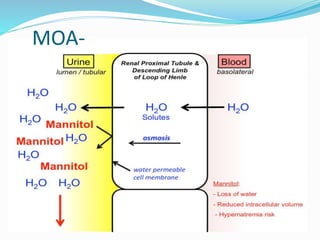

![MOA
carbonic anhydrase catalyzes formation of HCO3- and H+ from
H2O and CO2
inhibition of carbonic anhydrase decreases [H+] in tubule lumen
less H+ for for Na+/H+ exchange increased lumen Na+,
increased H2O retention](https://image.slidesharecdn.com/diuretics-190104065035/85/Diuretics-13-320.jpg)
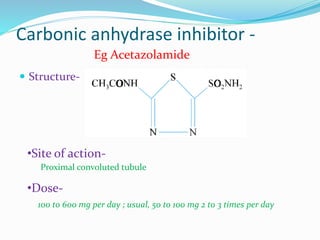
![USES-
used to treat chronic open-angle glaucoma aqueous humor
has high [HCO3-]
acute mountain sickness prevention and treatment
metabolic alkalosis
mostly used in combination with other diuretics in
resistant patients
SIDE EFFECTS-
rapid tolerance
increased HCO3- excretion causes metabolic acidosis
Drowsiness,fatigue,CNS depression
paresthesia (pins and needles under skin)
nephrolithiasis (renal stones)
K+ wasting](https://image.slidesharecdn.com/diuretics-190104065035/85/Diuretics-14-320.jpg)

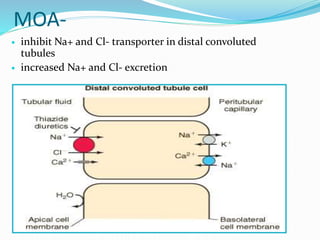


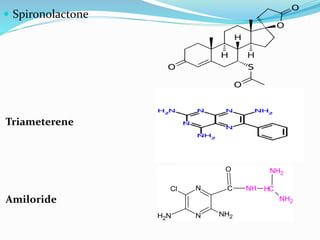

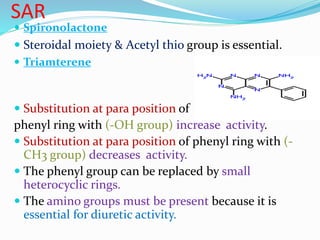
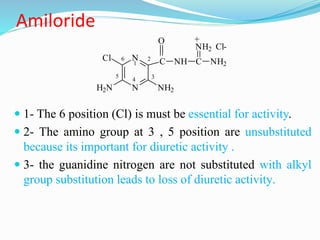
![Uses-
•primary hyperaldosteronism (adrenal adenoma, bilateral
adrenal hyperplasia)
•congestive heart failure
•cirrhosis
•nephrotic syndrome
•in conjunction with K+ wasting diuretics
Side Effects
•hyperkalemia: monitor plasma [K+]
•spironolactone: gynecomastia
•triamterene: megaloblastic anemia in cirrhosis patients
•amiloride: increase in blood urea nitrogen, glucose intolerance in
diabetes mellitus](https://image.slidesharecdn.com/diuretics-190104065035/85/Diuretics-24-320.jpg)