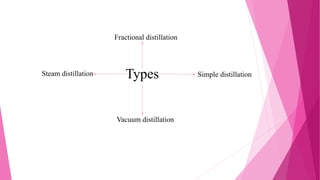
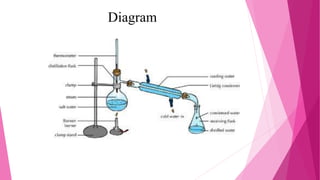
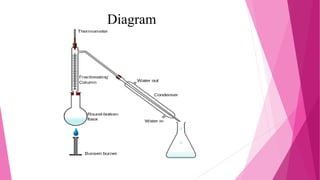
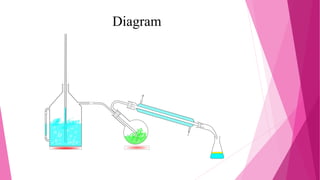
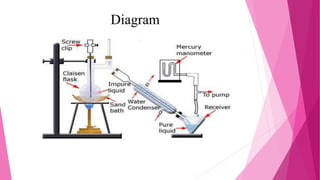
Distillation is a technique used to separate mixtures based on differences in boiling points. It works by heating the mixture to vaporize components, which are then cooled and condensed separately. There are several types of distillation including simple, fractional, steam, and vacuum distillation. Simple distillation is used when components have significantly different boiling points, while fractional distillation uses a column to separate closer-boiling components. Steam distillation isolates heat-sensitive materials, and vacuum distillation allows distilling higher-boiling mixtures. Distillation has many commercial uses such as producing fuels, solvents, and separating air into its components.