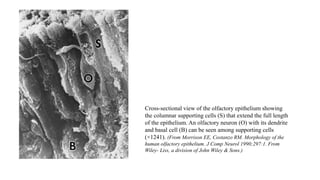
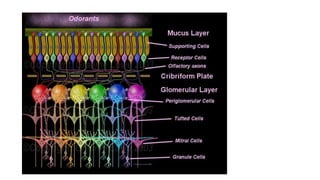
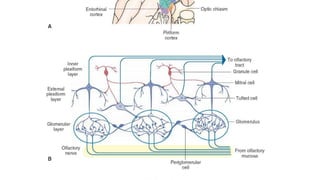
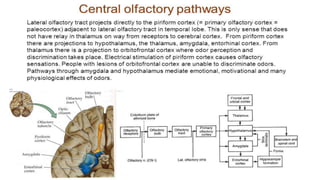
1. Disorders of olfaction can be conductive, resulting from nasal obstruction, or sensorineural, due to damage to the olfactory neuroepithelium.
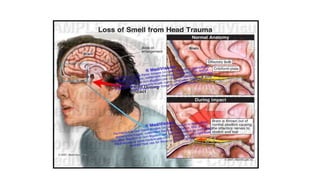
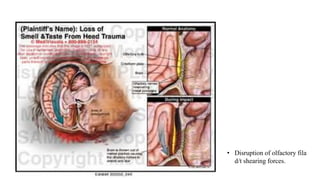
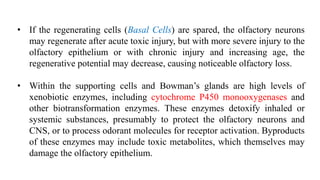
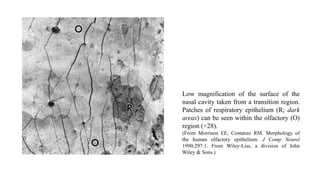
2. Common causes of sensorineural disorders include upper respiratory infections, head trauma, tumors near the olfactory region, congenital defects, toxins, age-related changes, and neurodegenerative diseases.
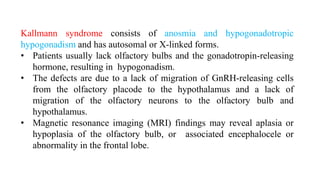
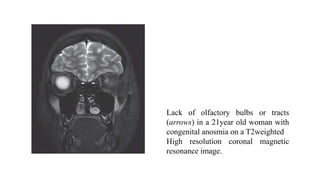
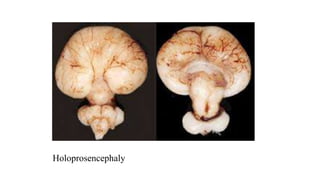
3. Specific conditions that can cause olfactory disorders are post-viral olfactory dysfunction, Kallmann syndrome, septo-optic dysplasia, holoprosencephaly, exposure to metals or other toxins, Alzheimer's disease, Parkinson's disease, and epilepsy presenting with olfactory auras.