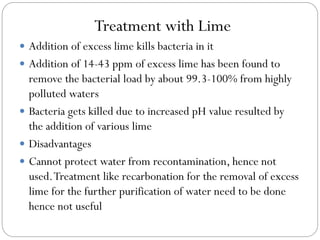
This document summarizes different methods of disinfecting water, including chlorination, ozonation, treatment with lime, iodine, bromine, ultraviolet rays, potassium permanganate, silver, and boiling. It provides details on the chemical processes involved, advantages, and disadvantages of each method. Chlorination is described as the most commonly used and effective method, involving the addition of chlorine to water to form hypochlorous acid and hypochlorite ions which kill bacteria. The ideal pH for chlorination is below 7 to maximize the amount of hypochlorous acid present.