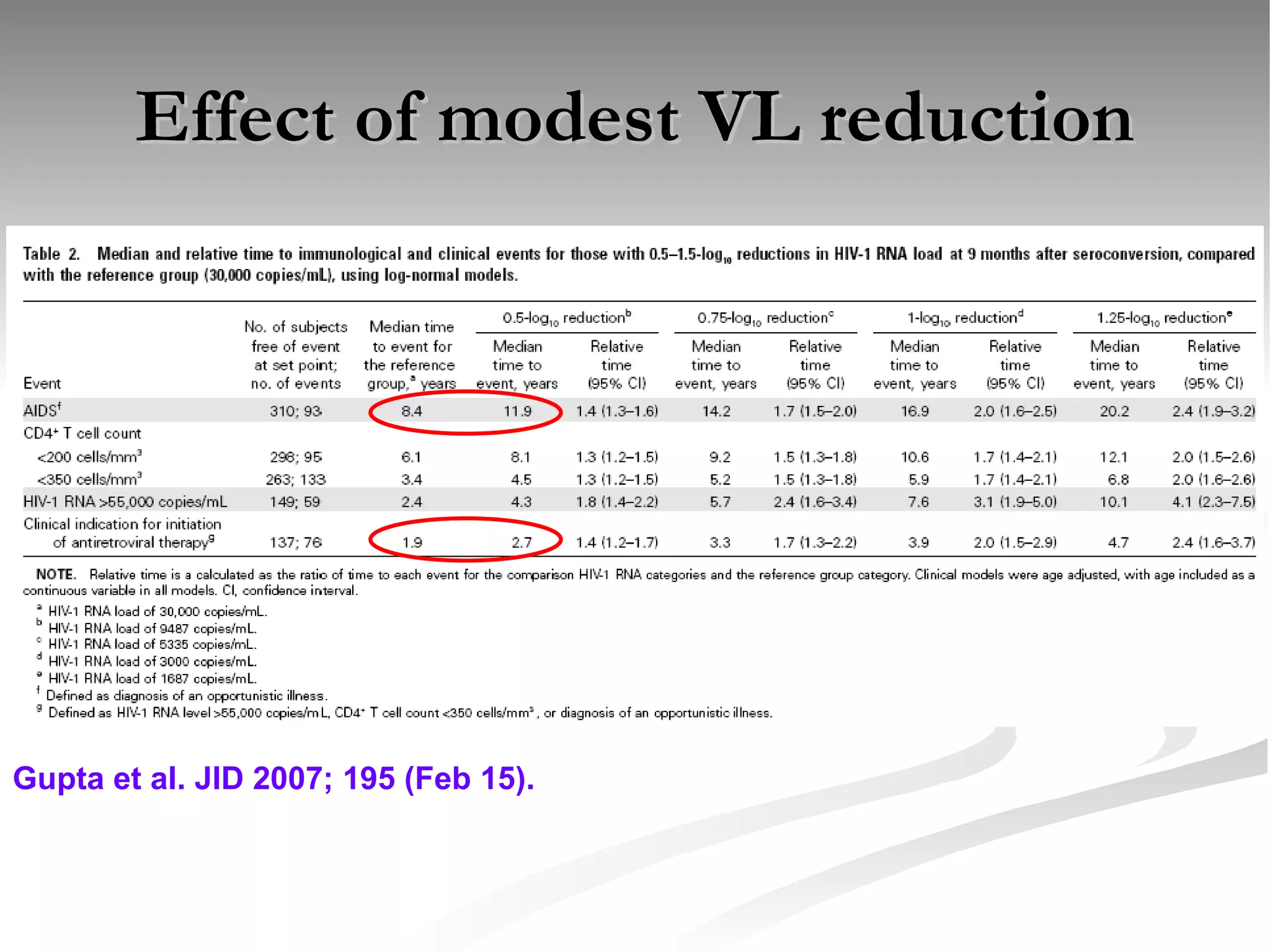
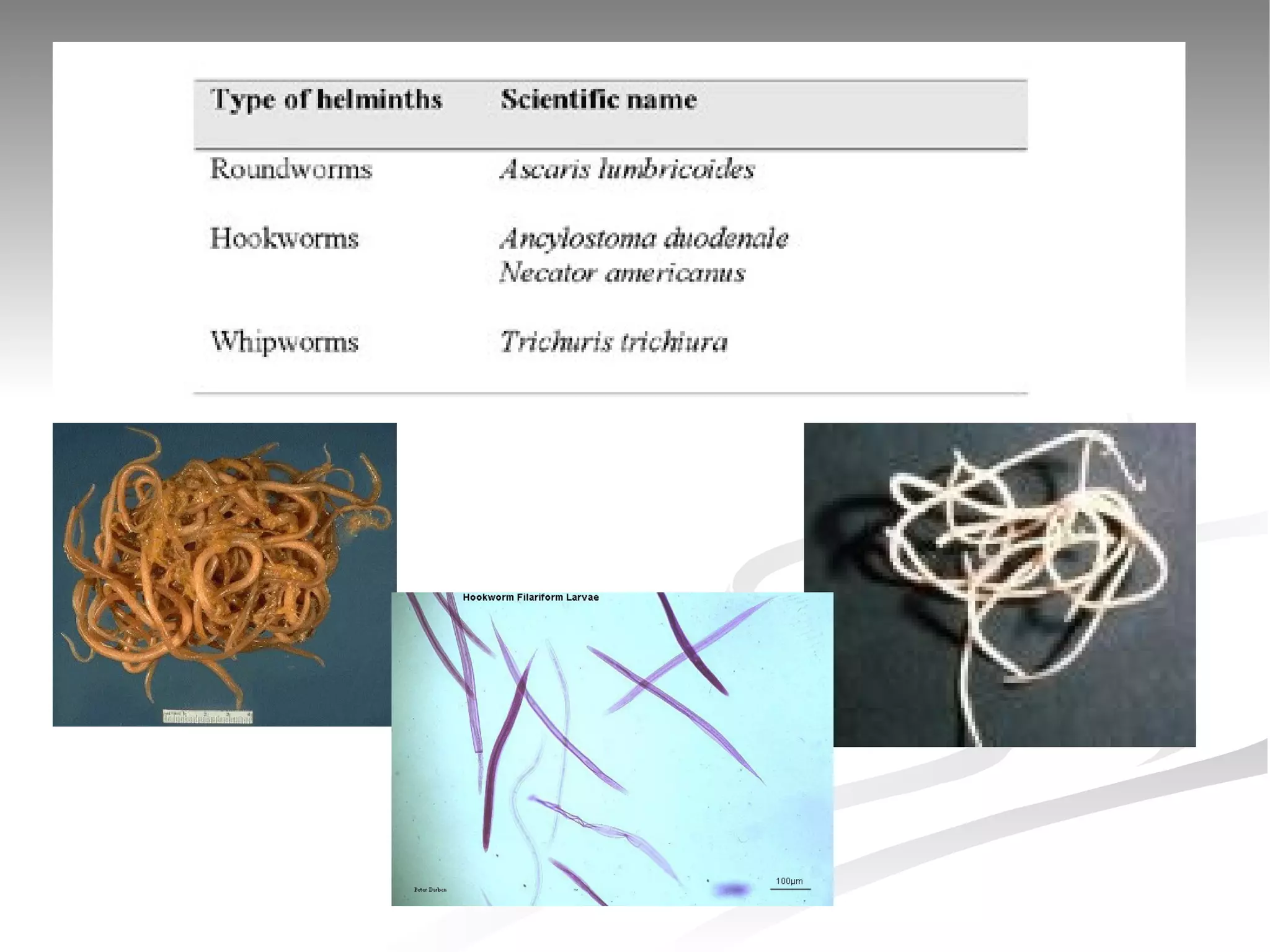
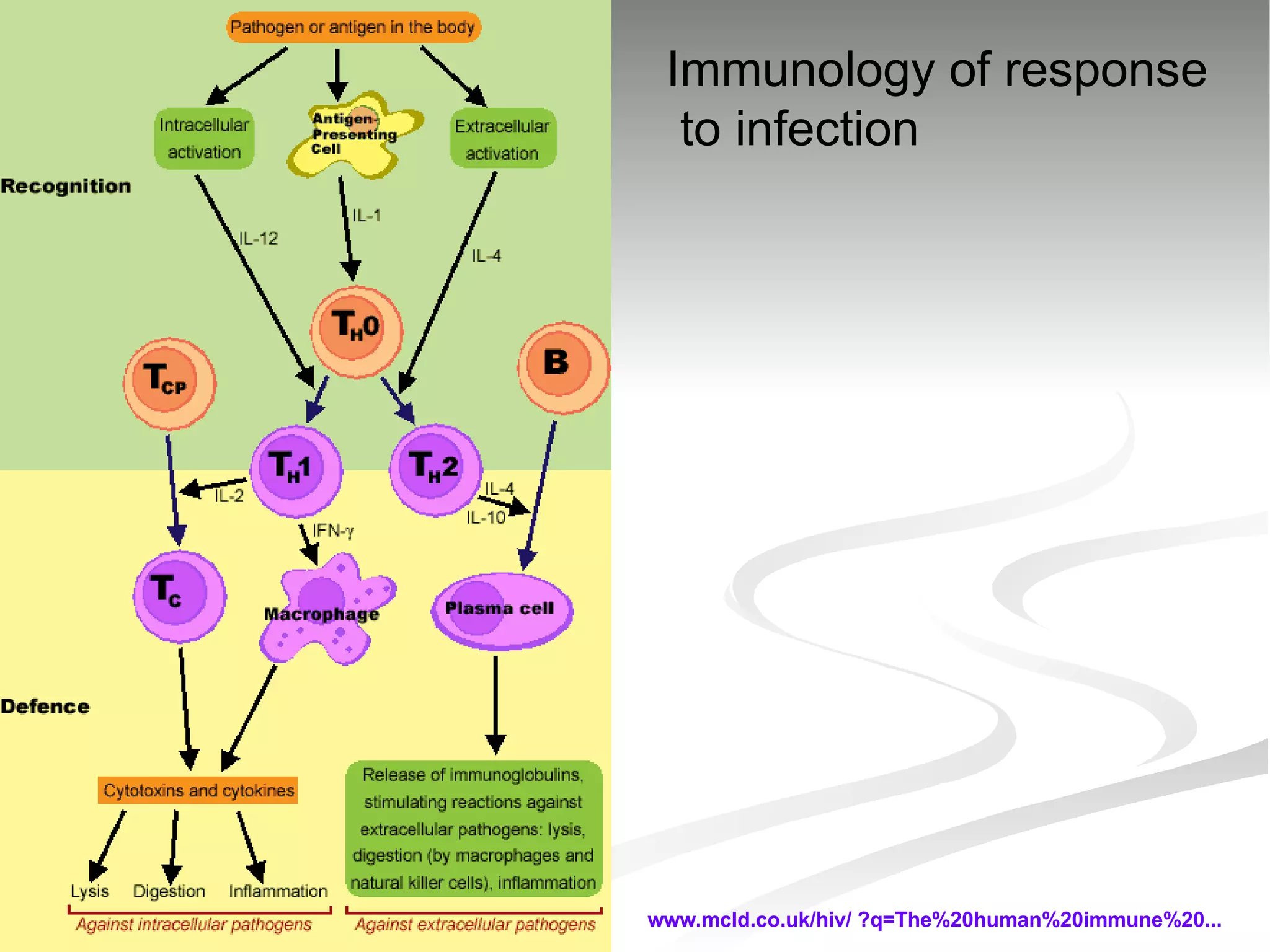
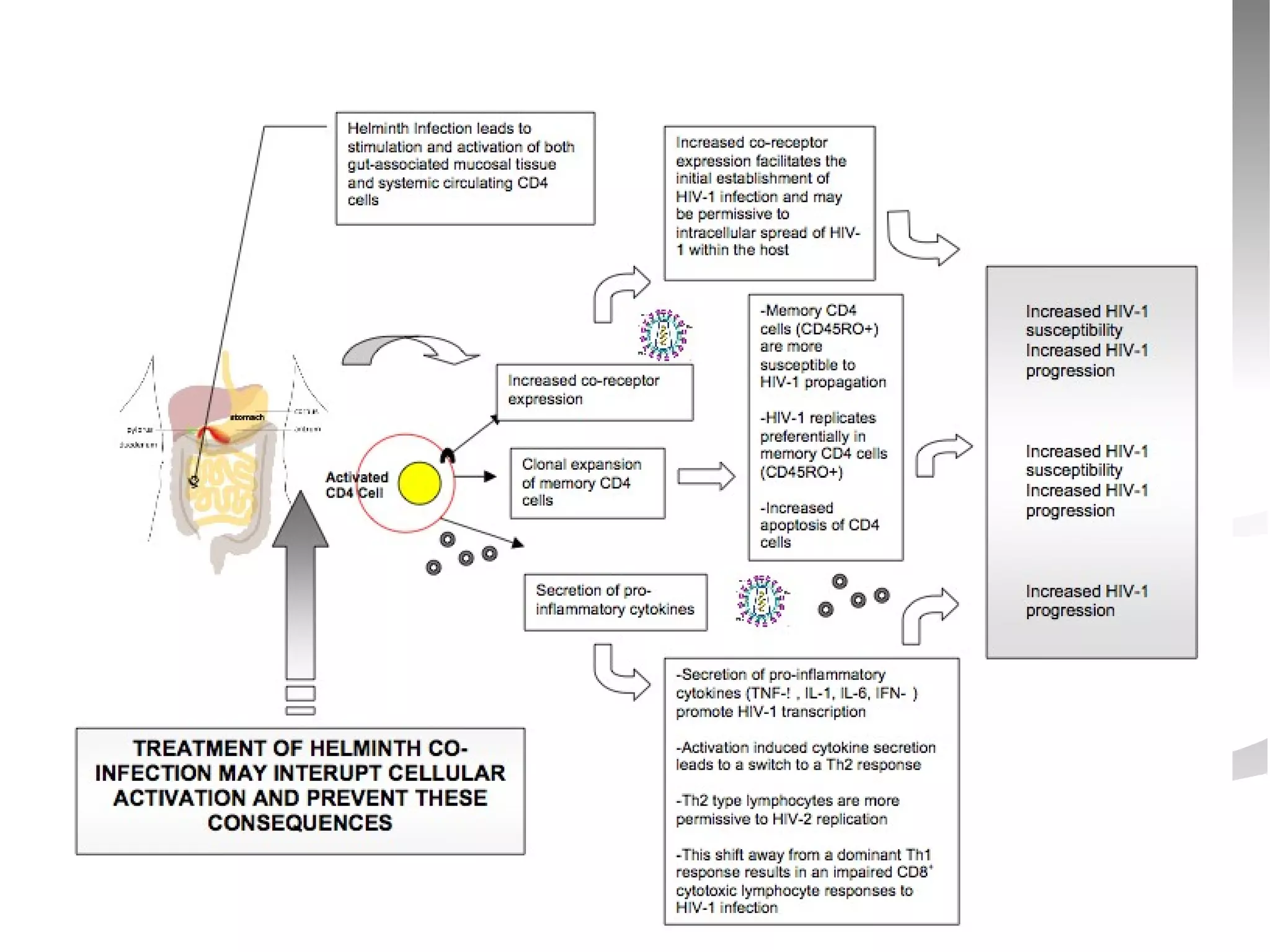
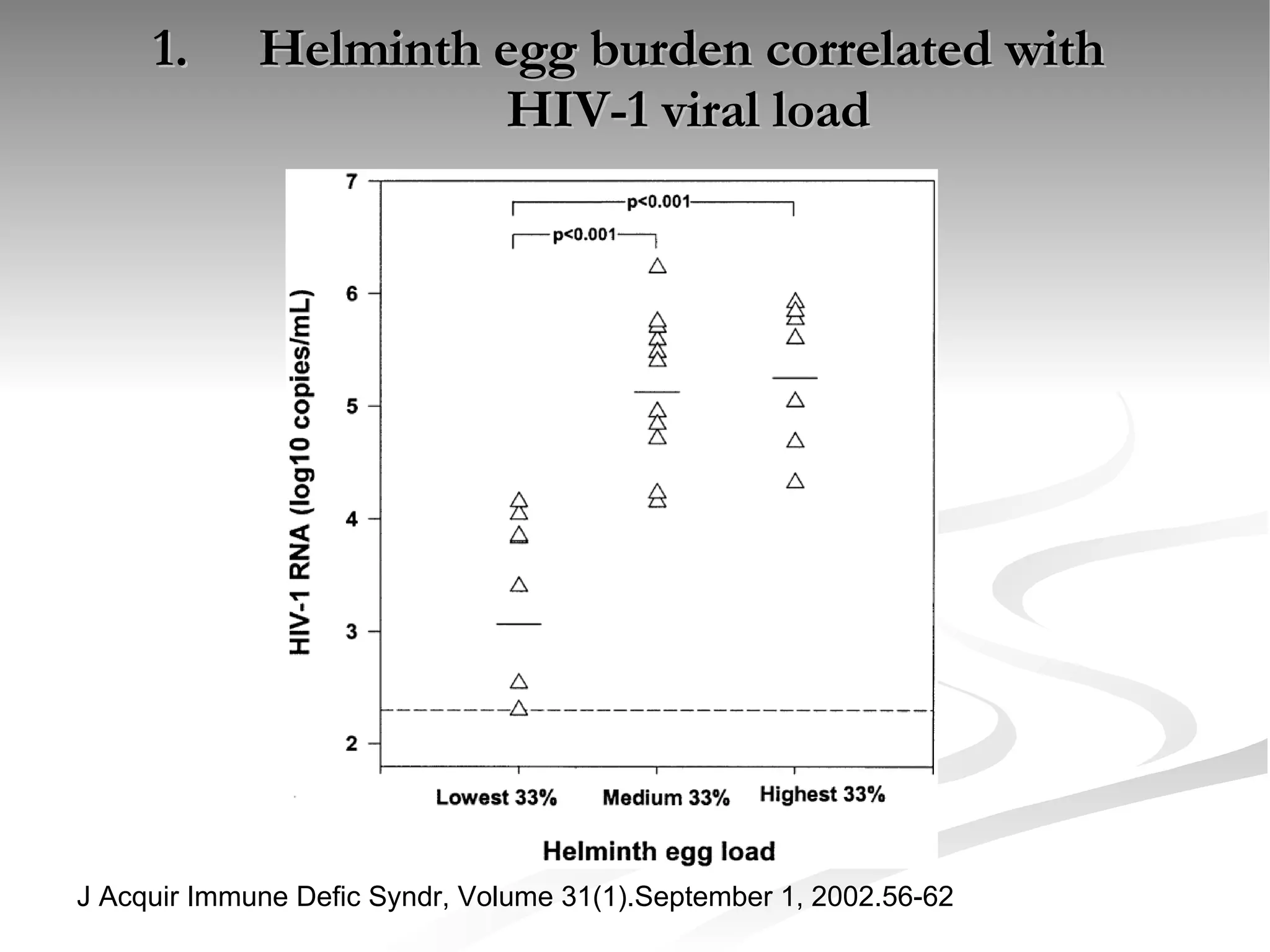
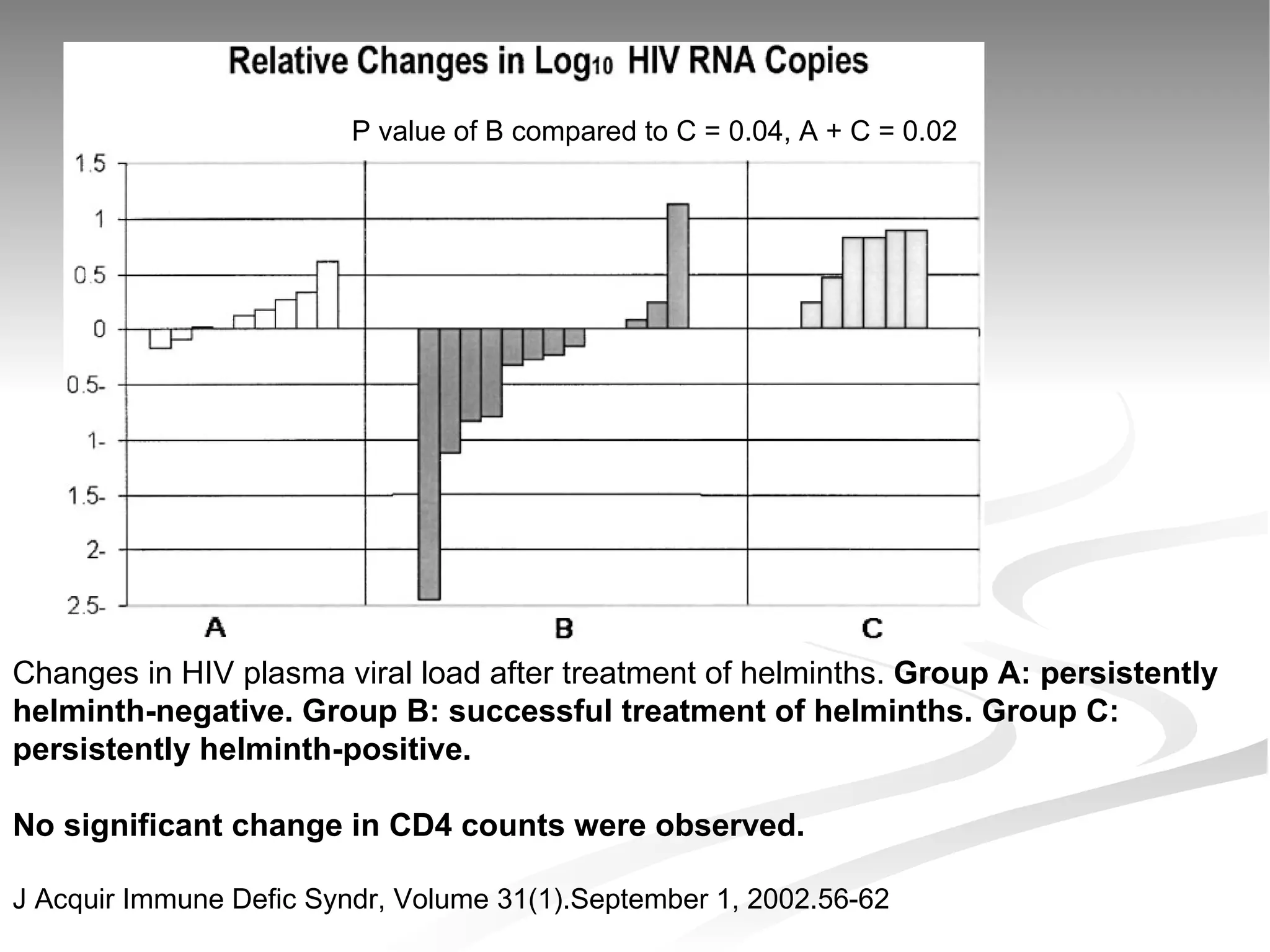
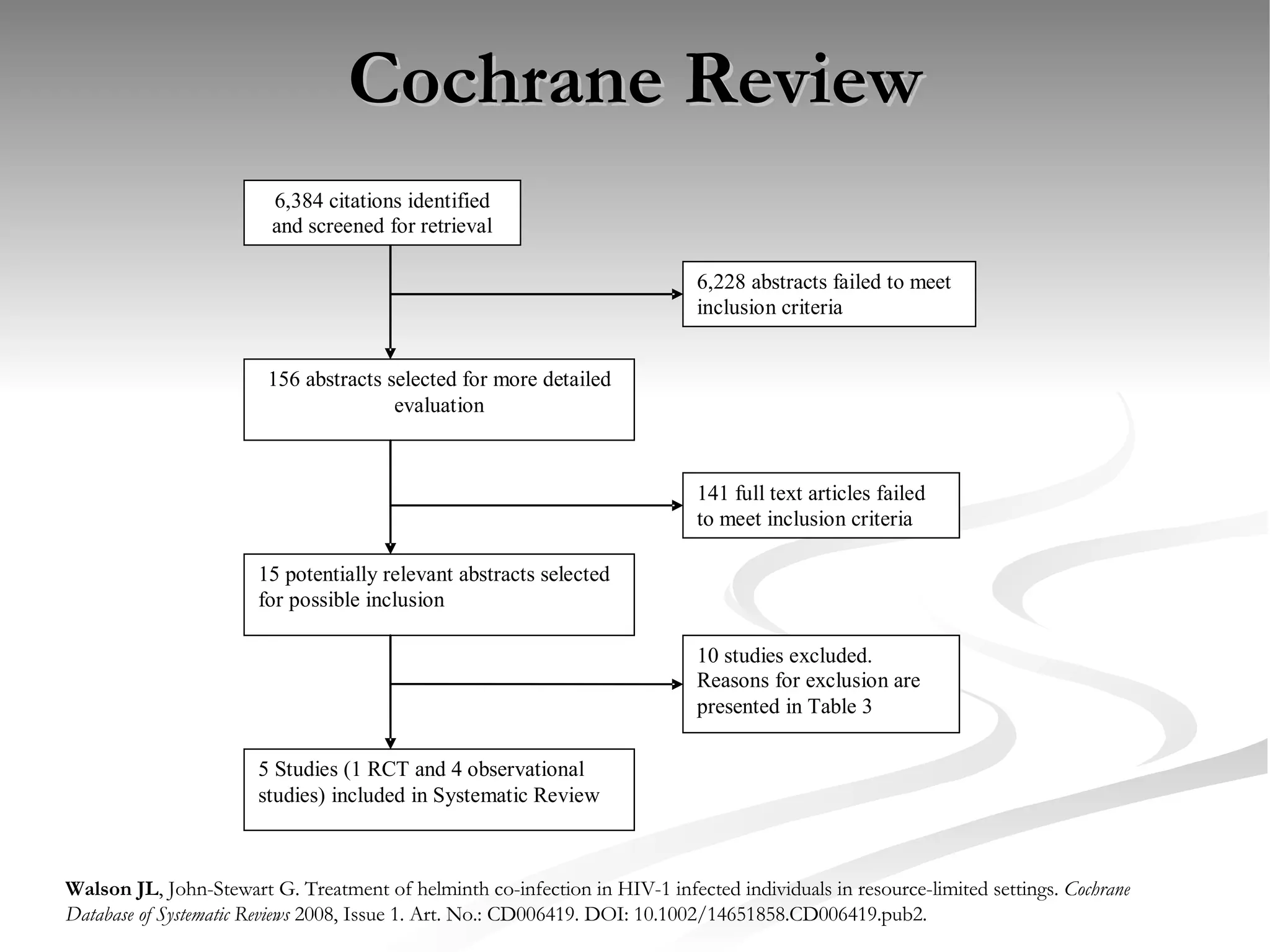
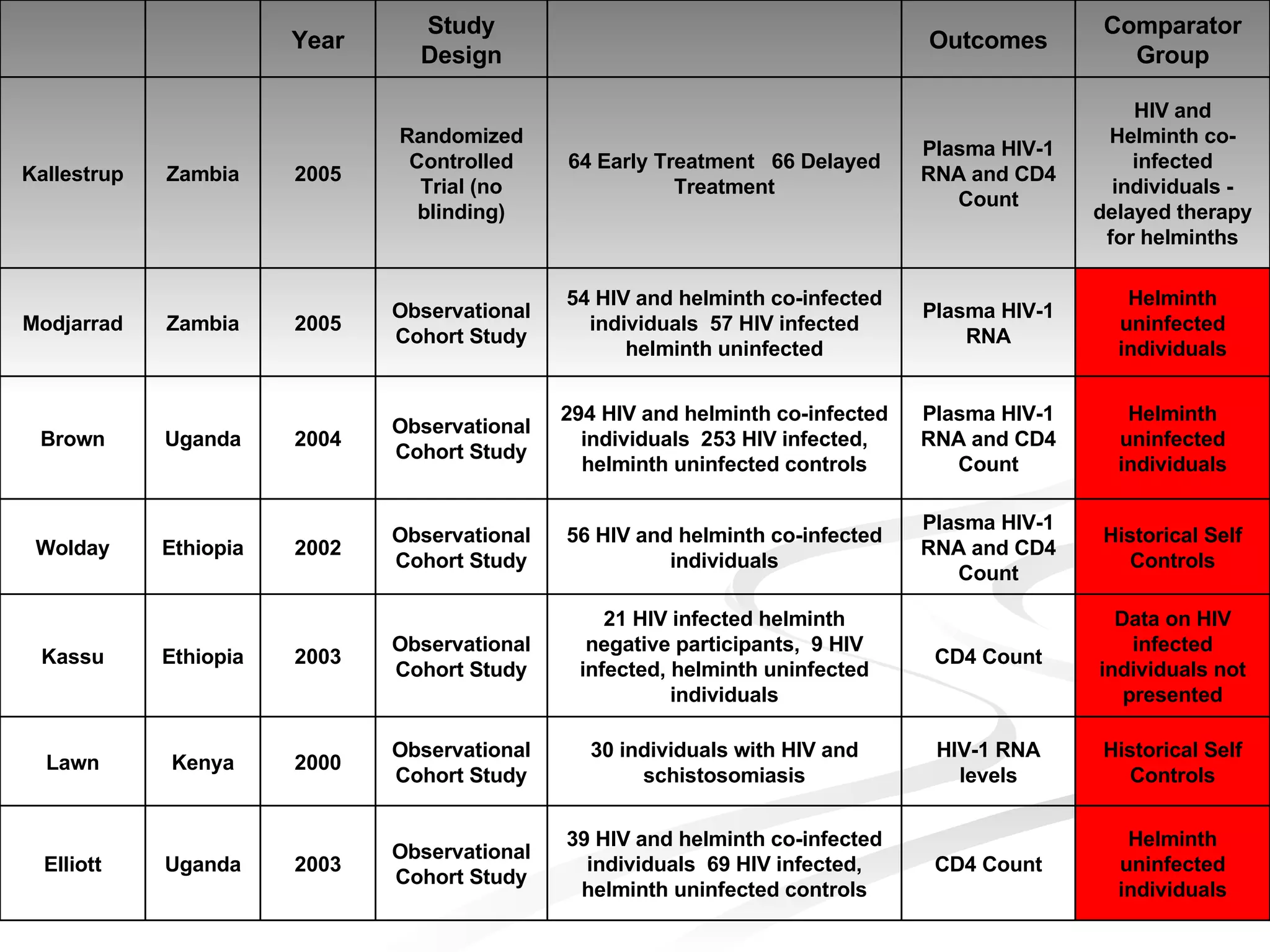
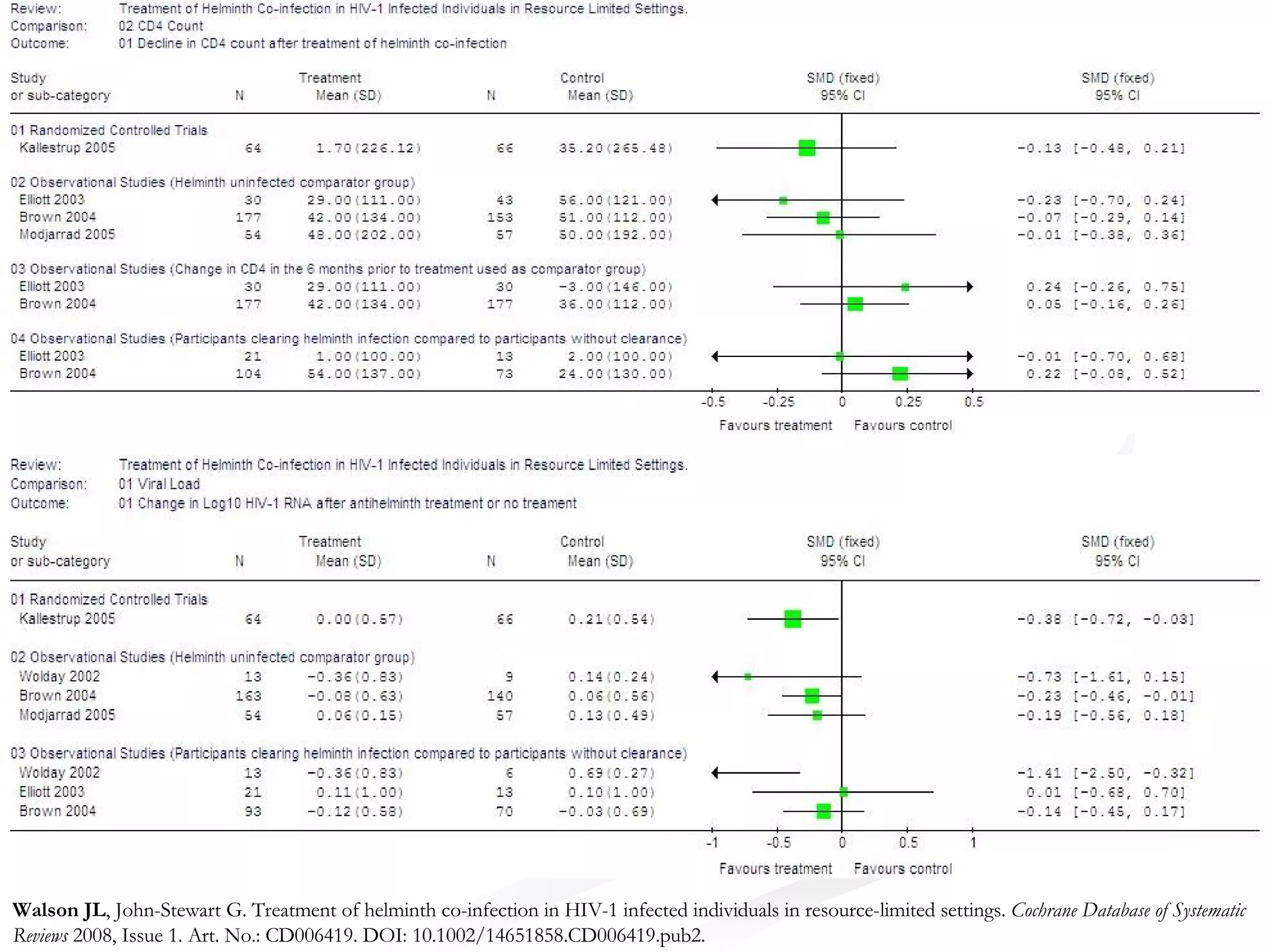
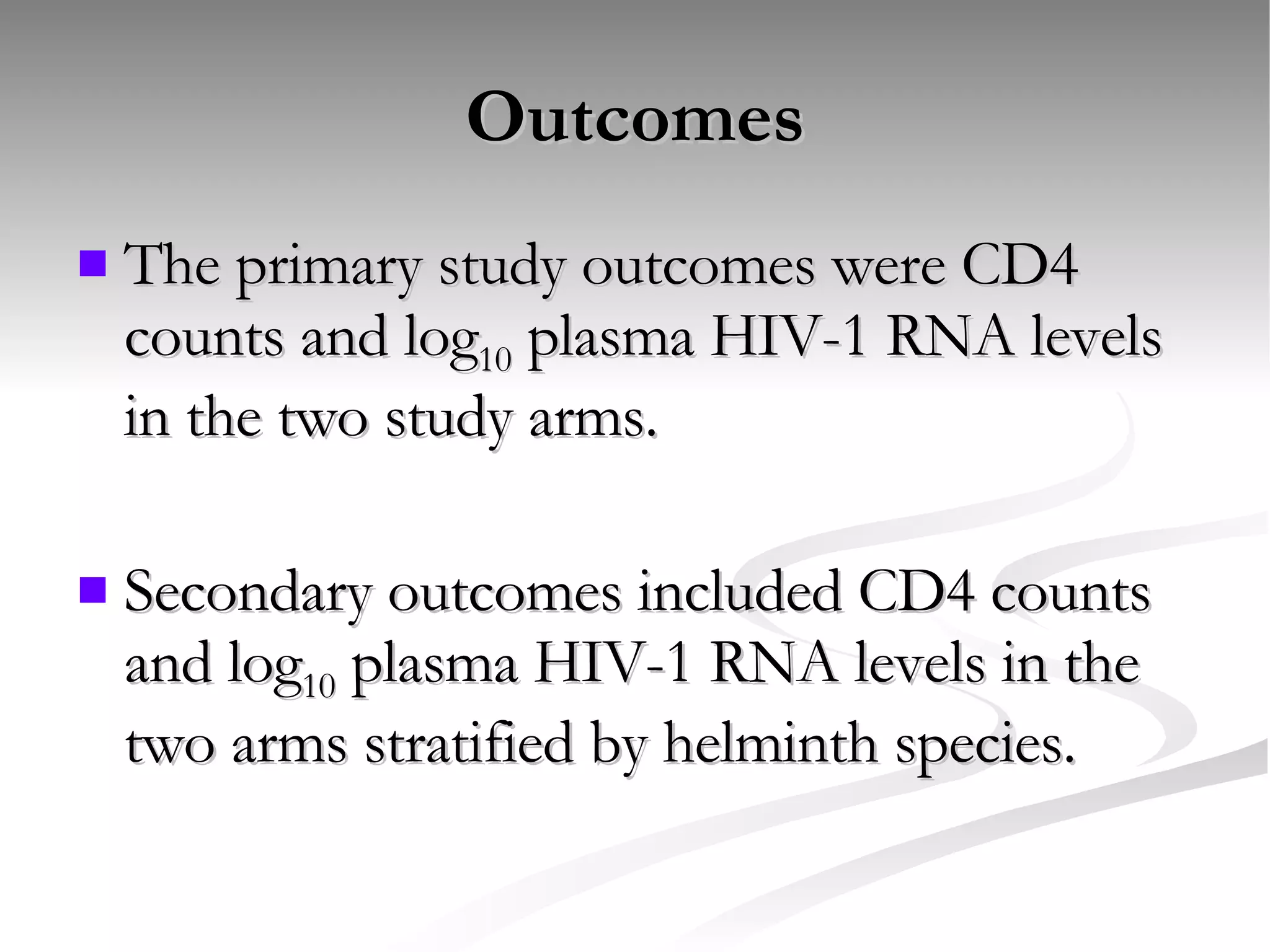
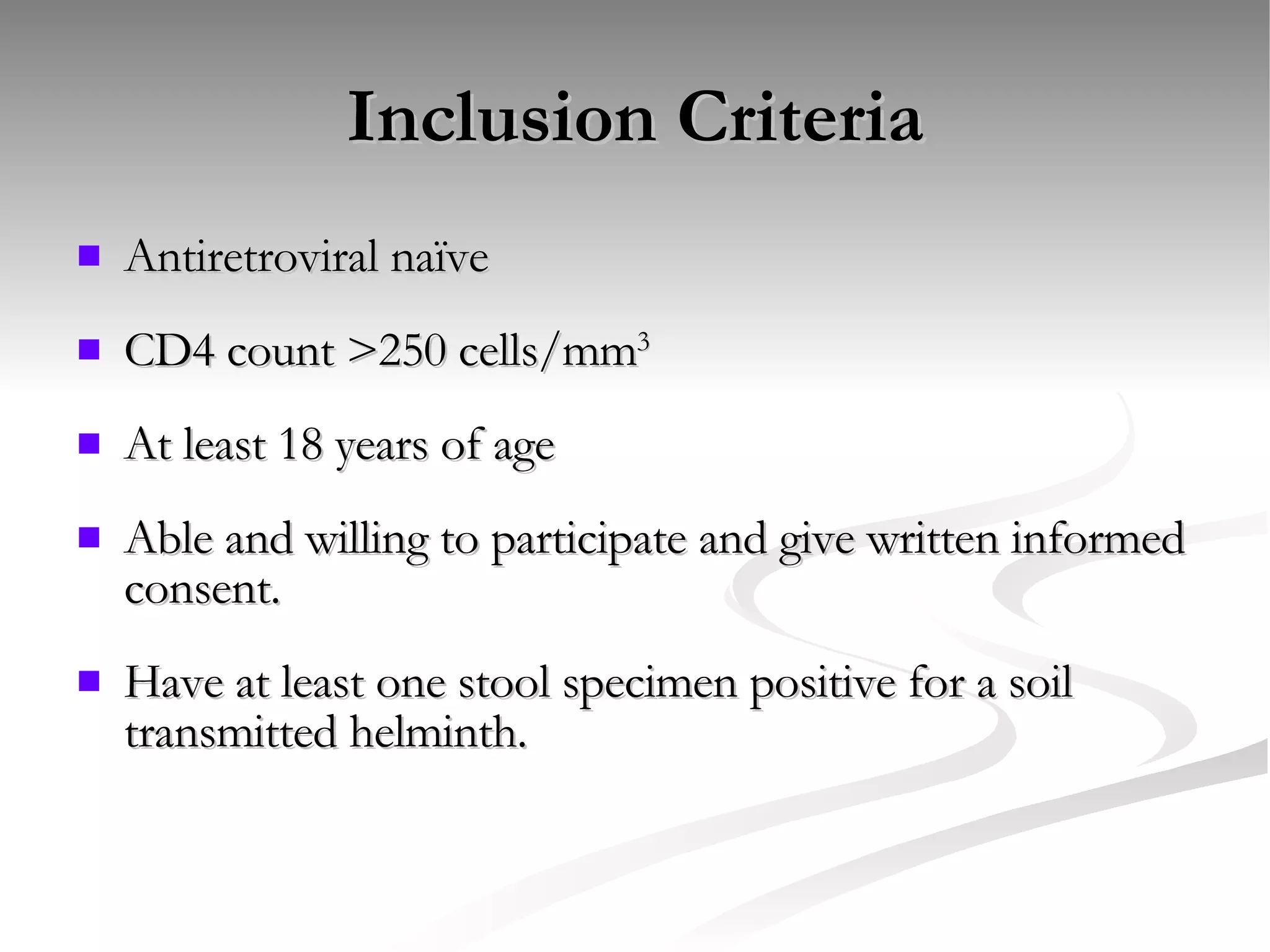
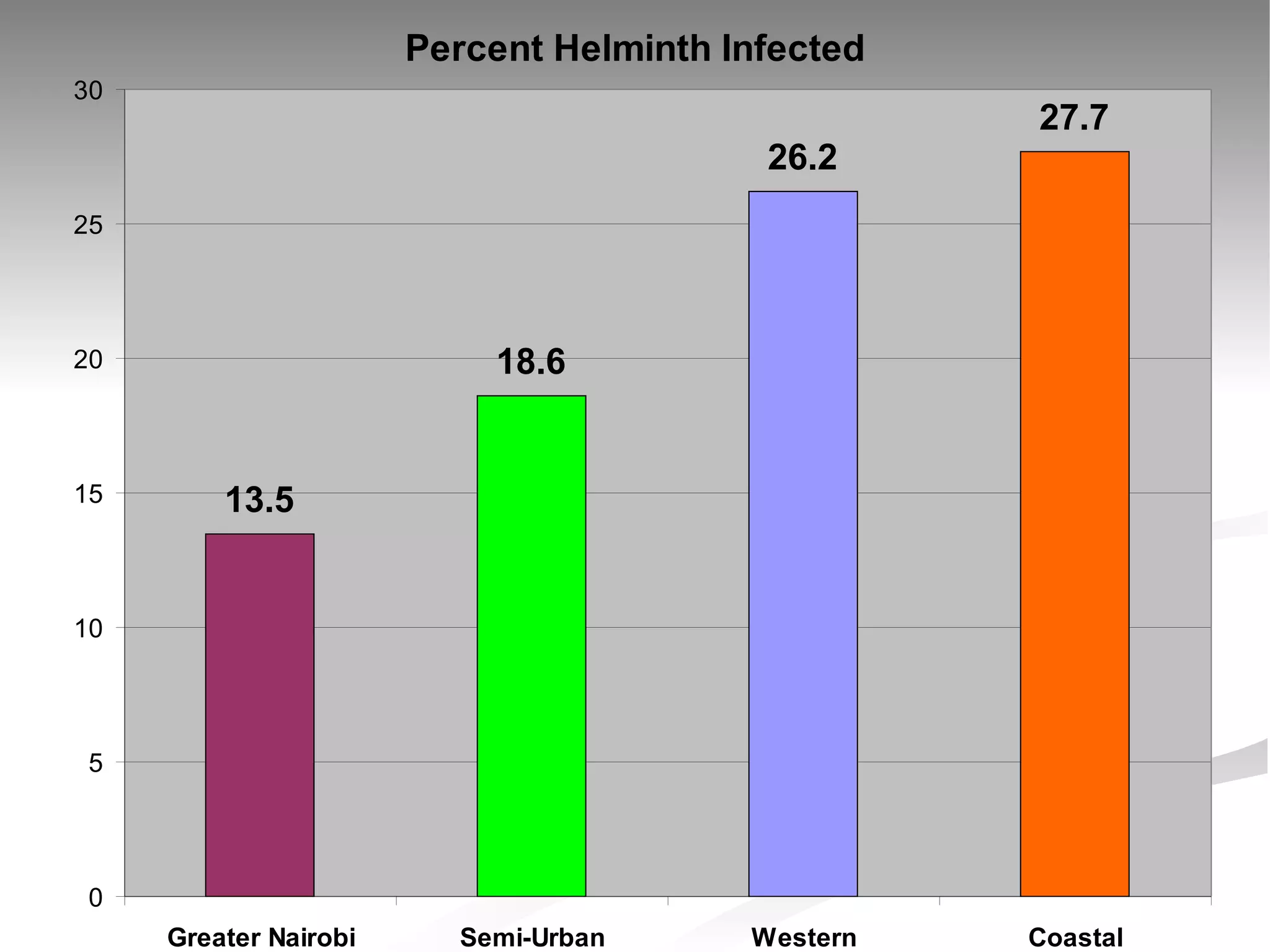
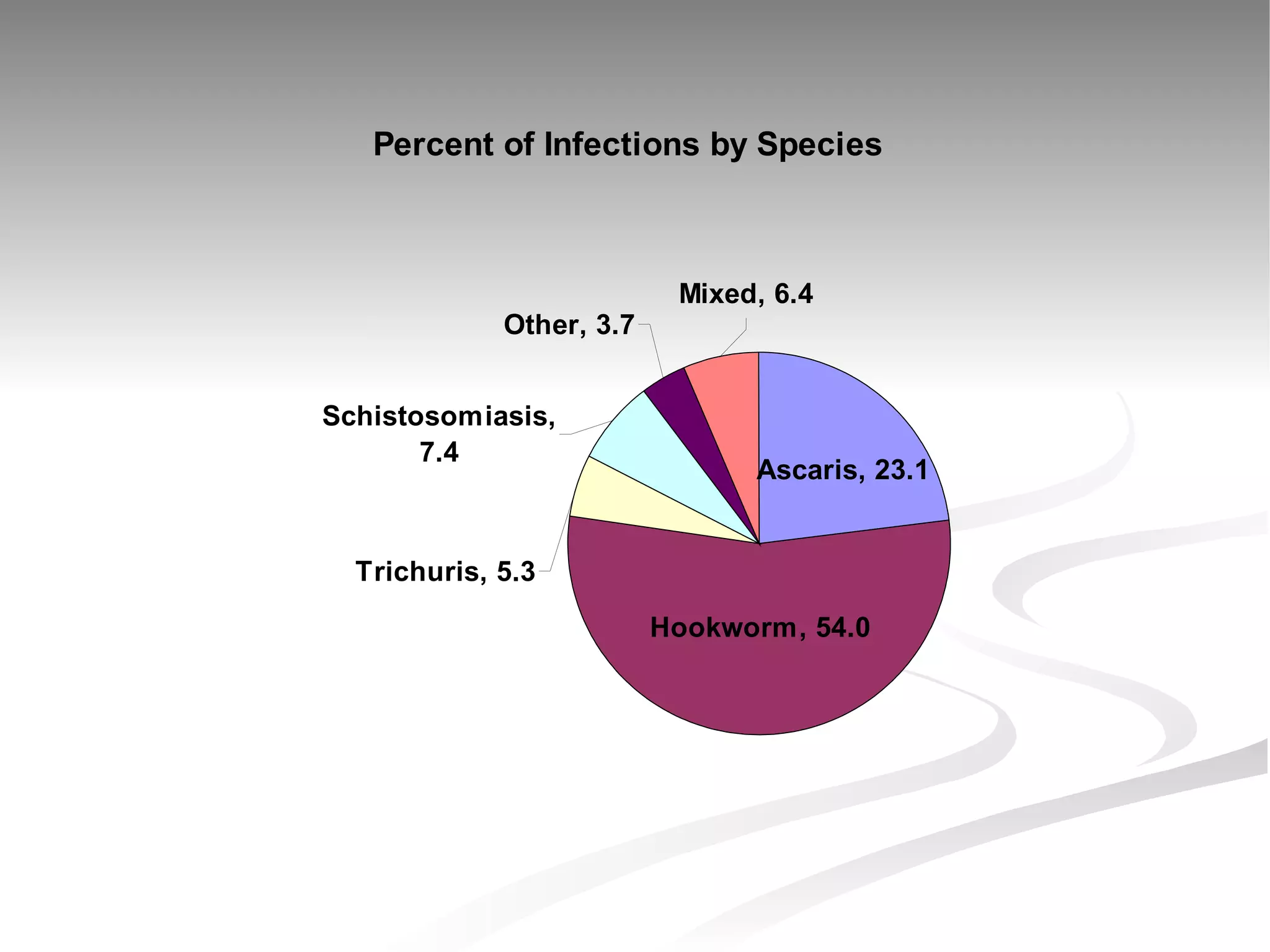
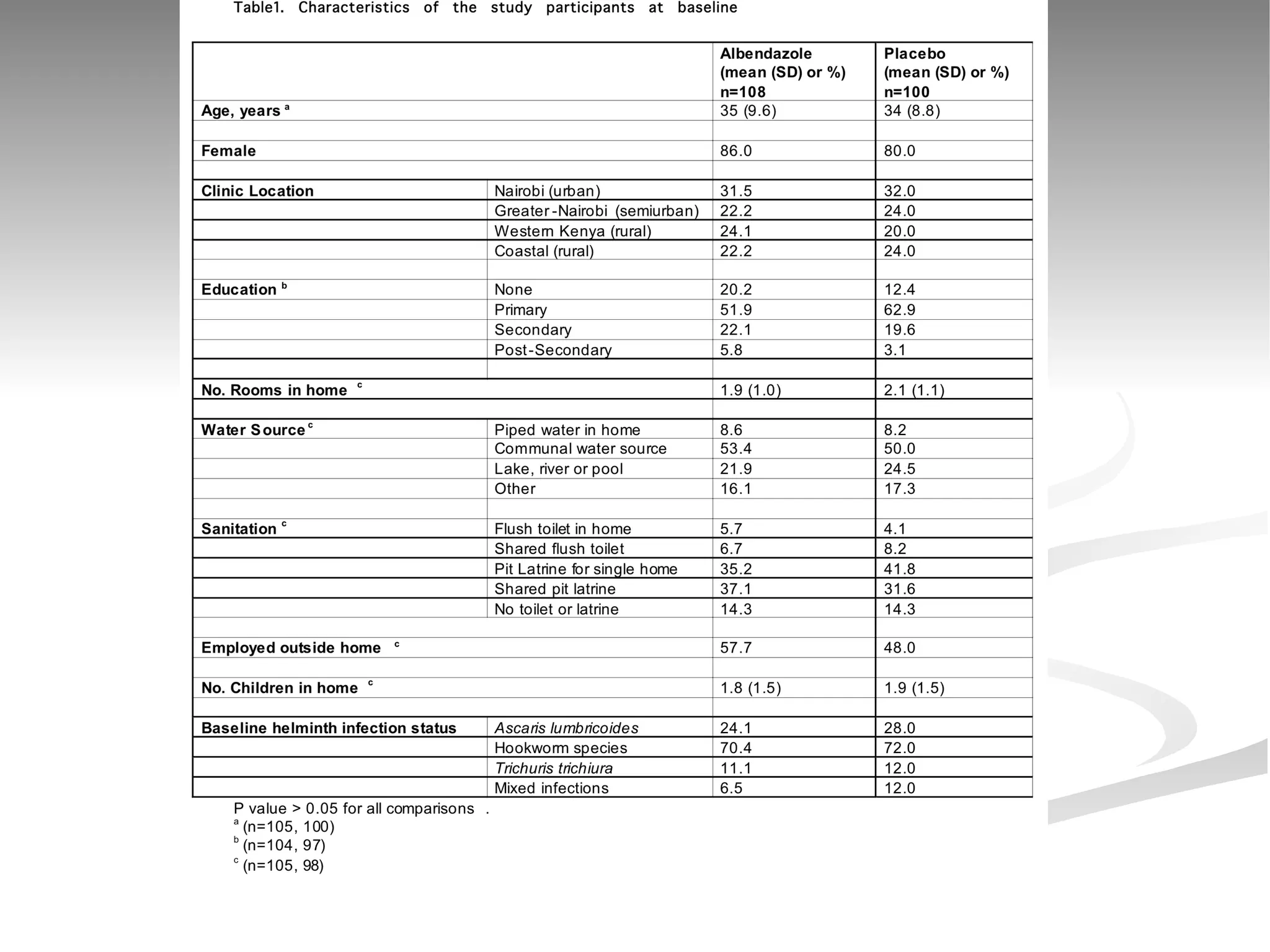
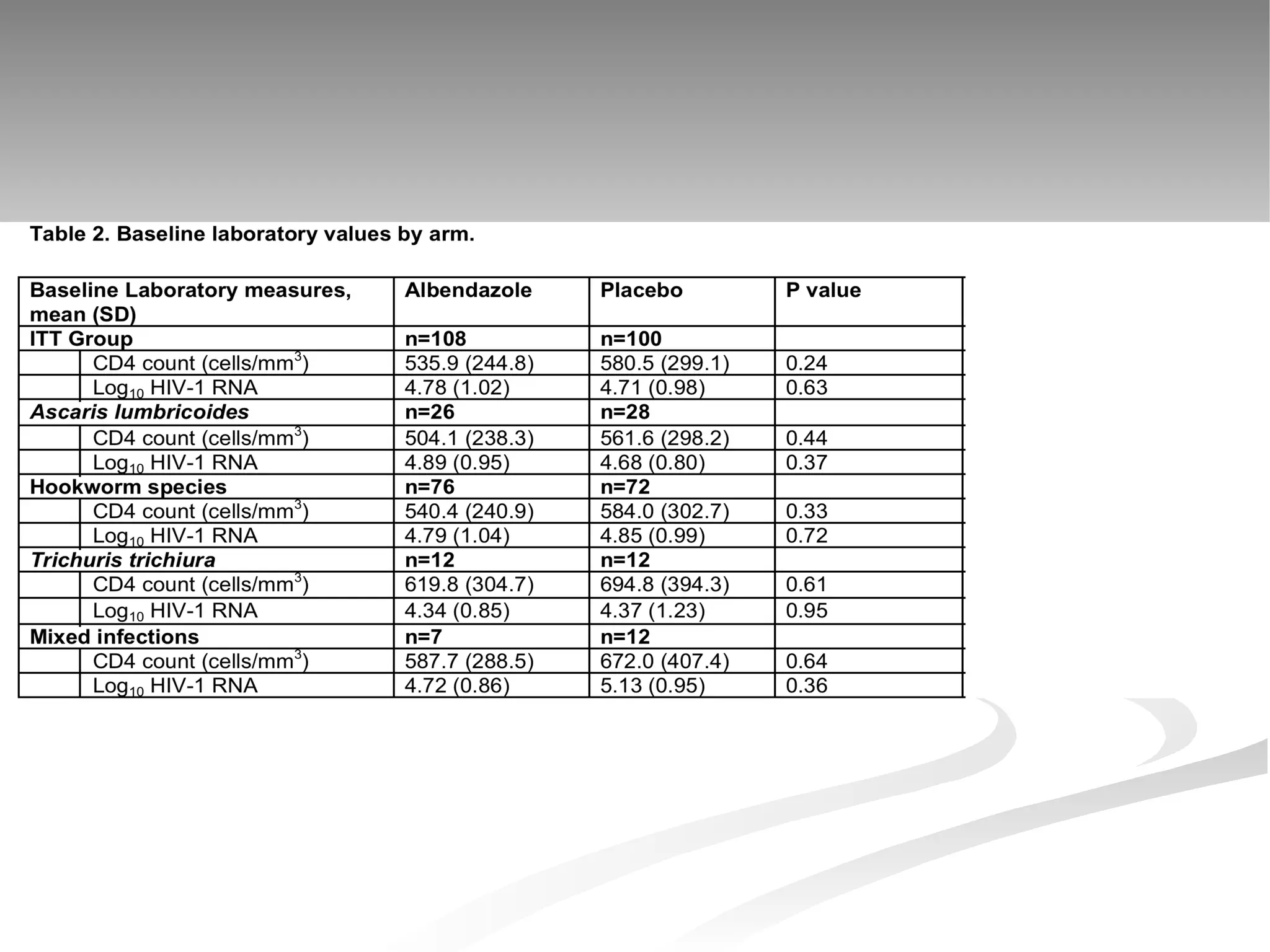
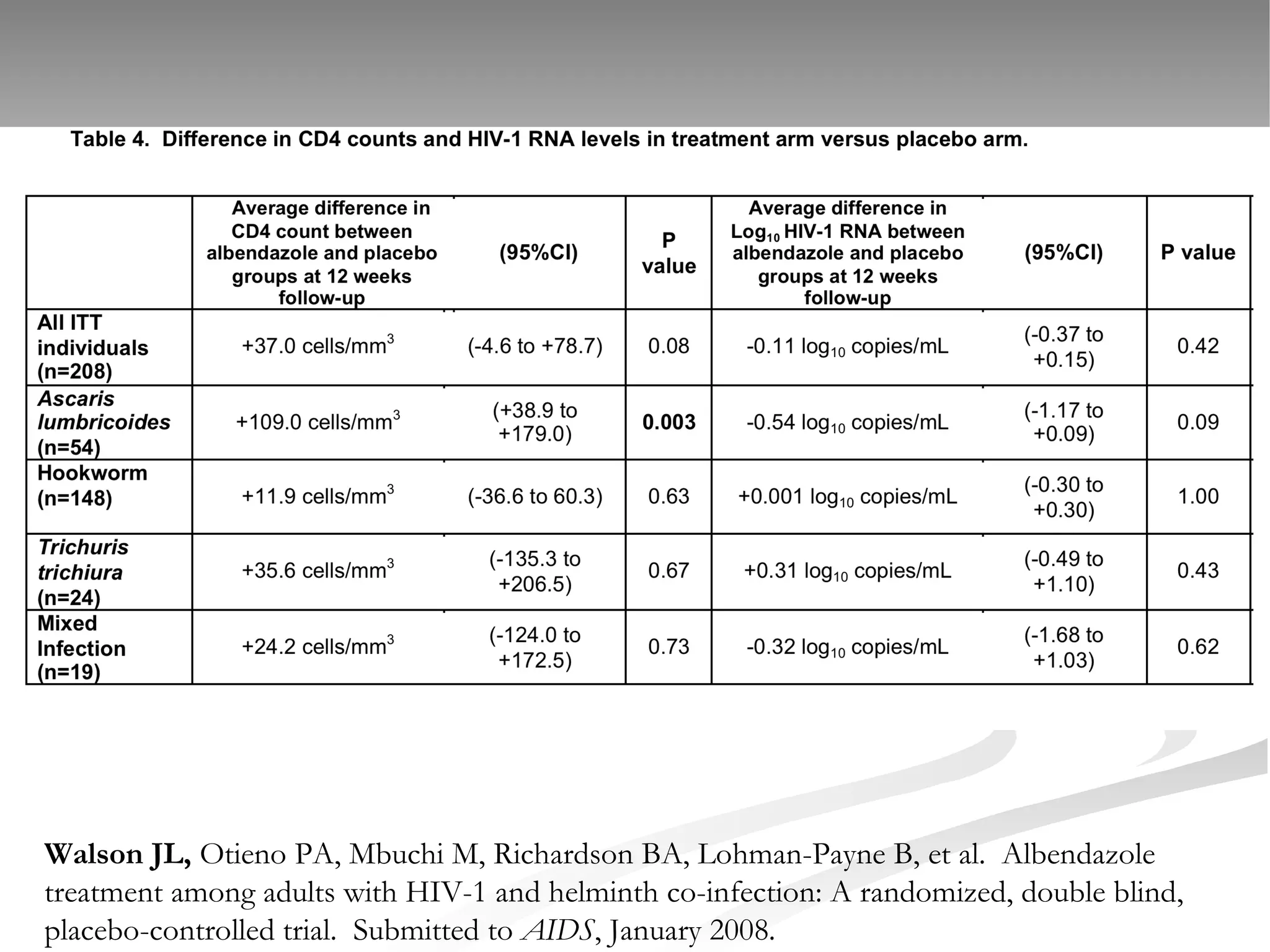
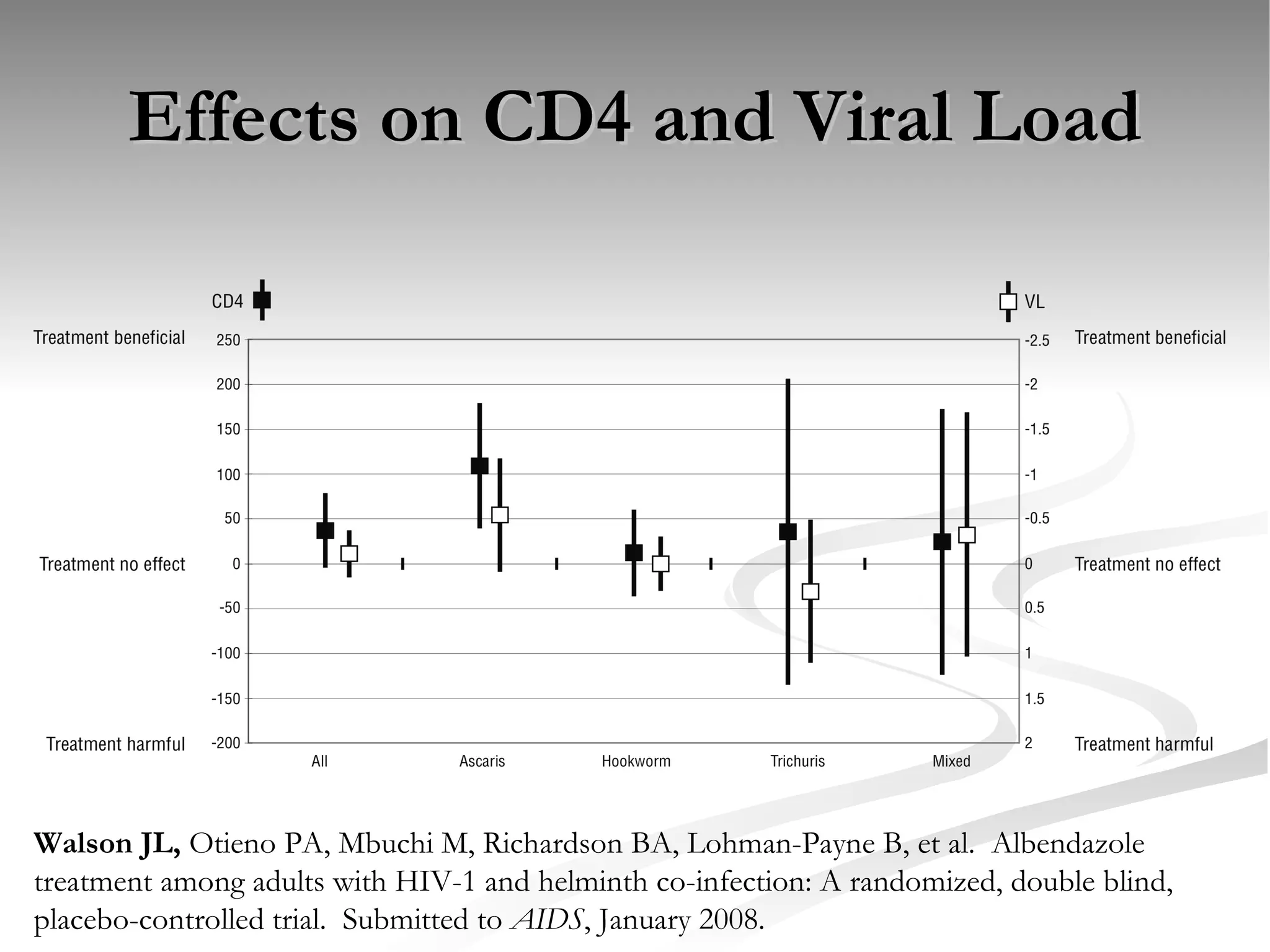
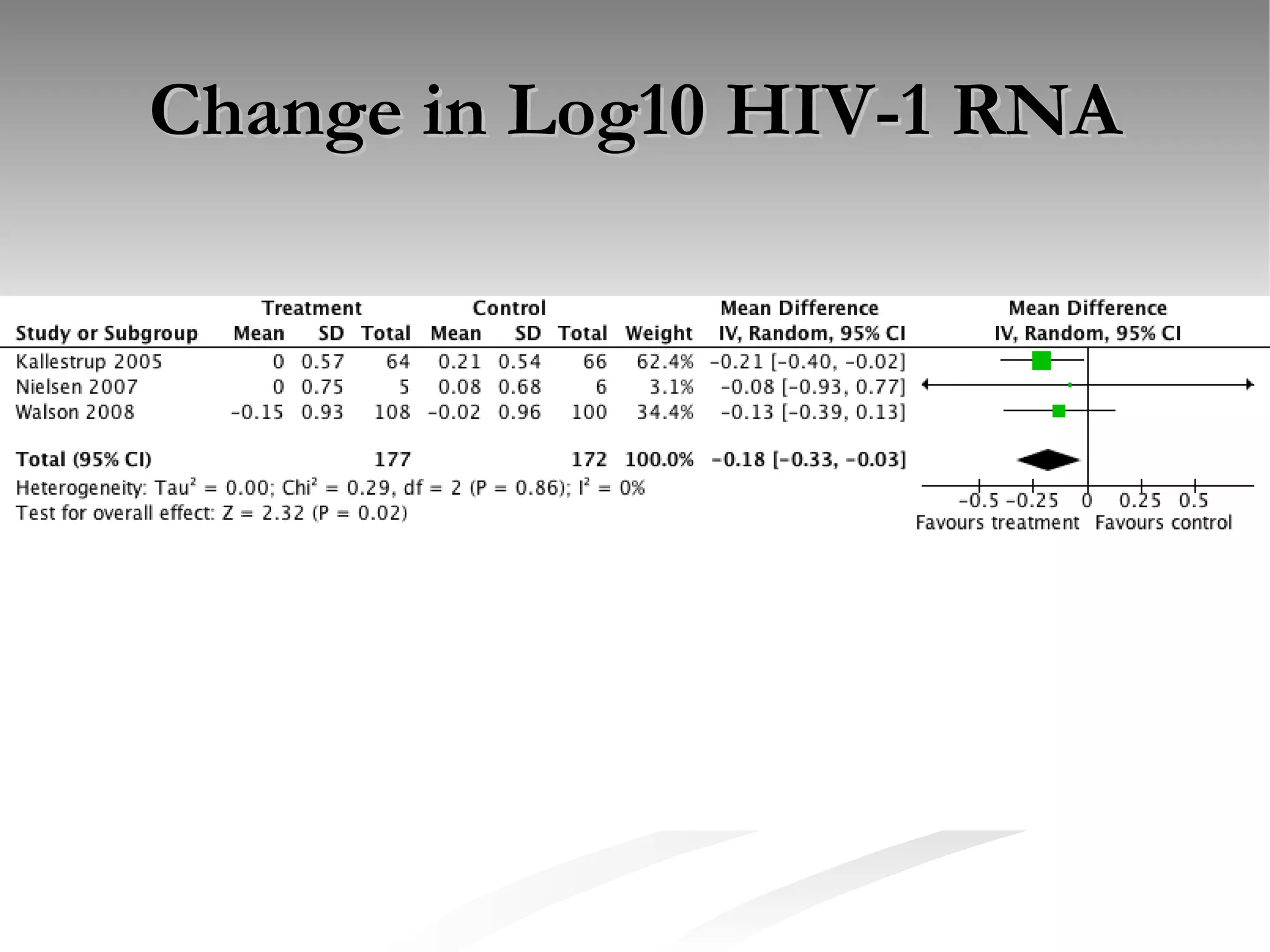
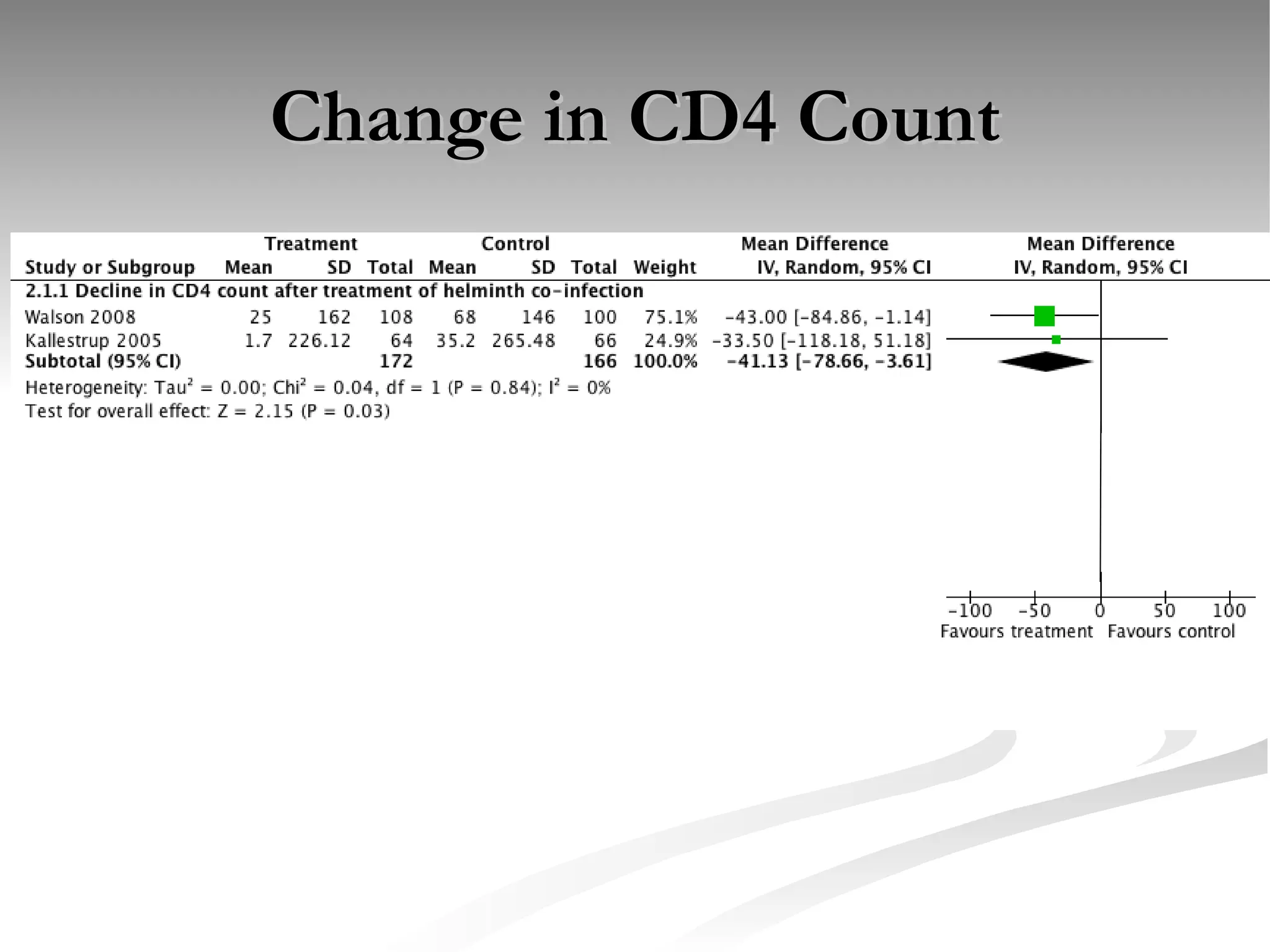
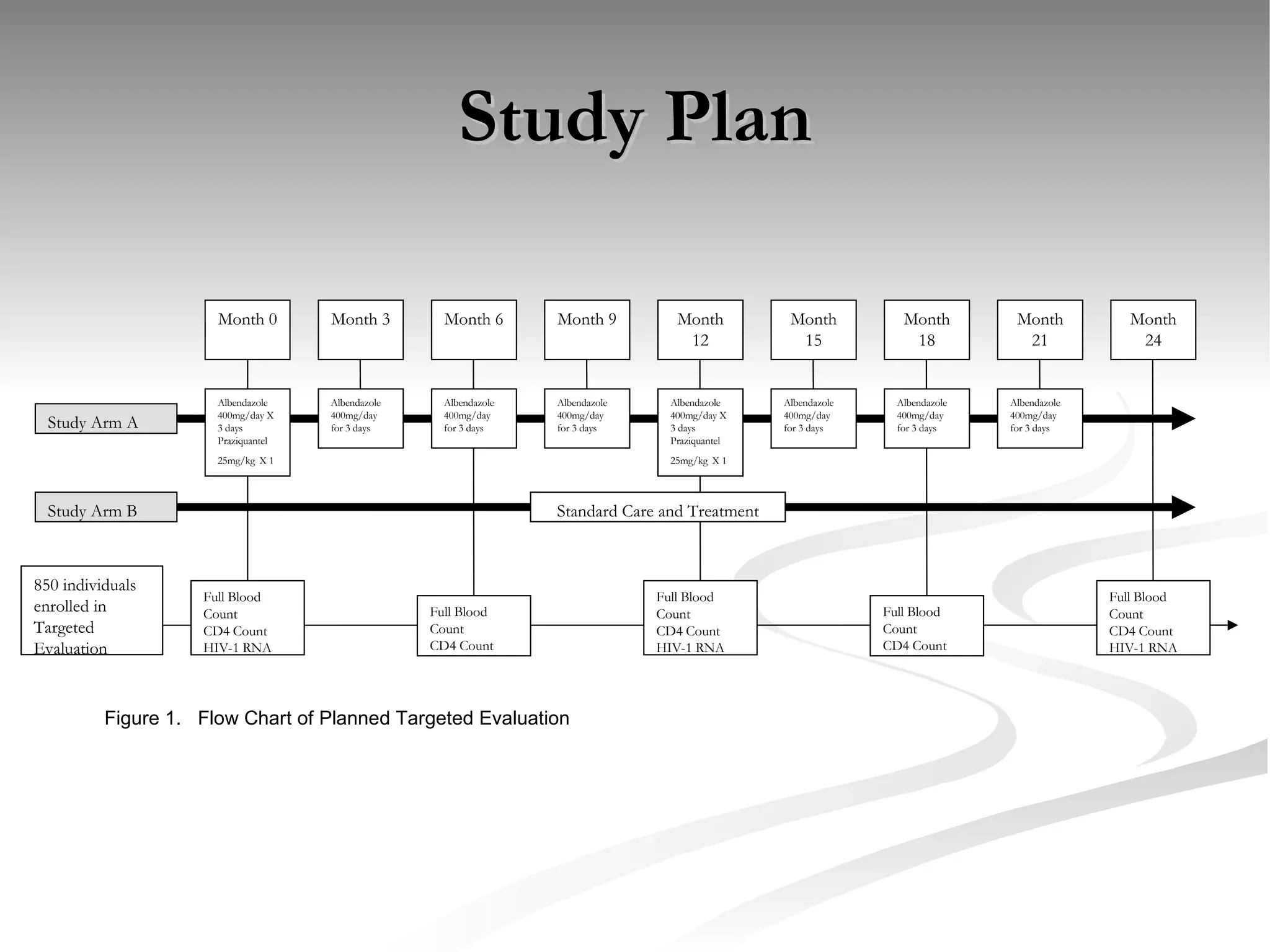
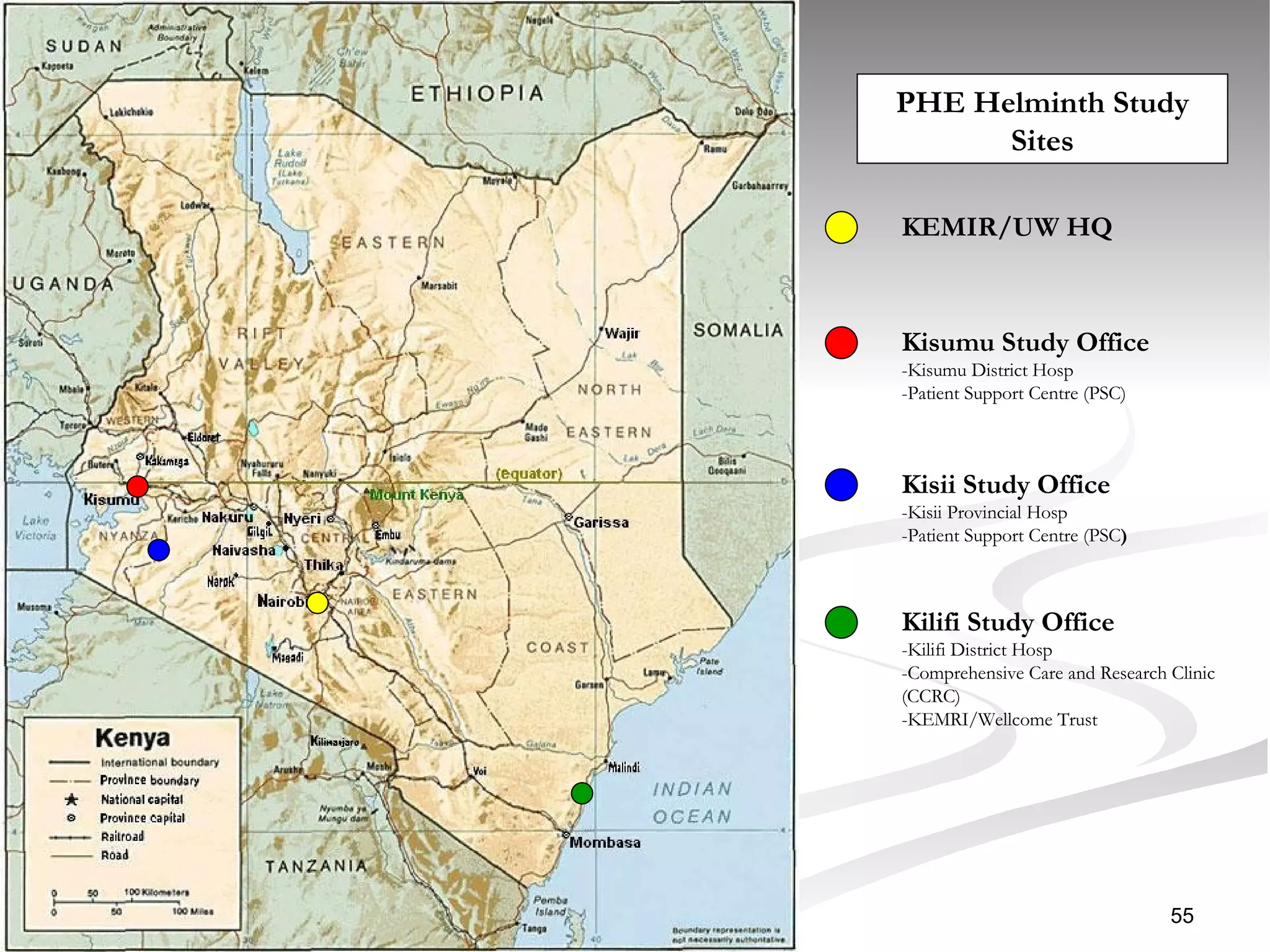
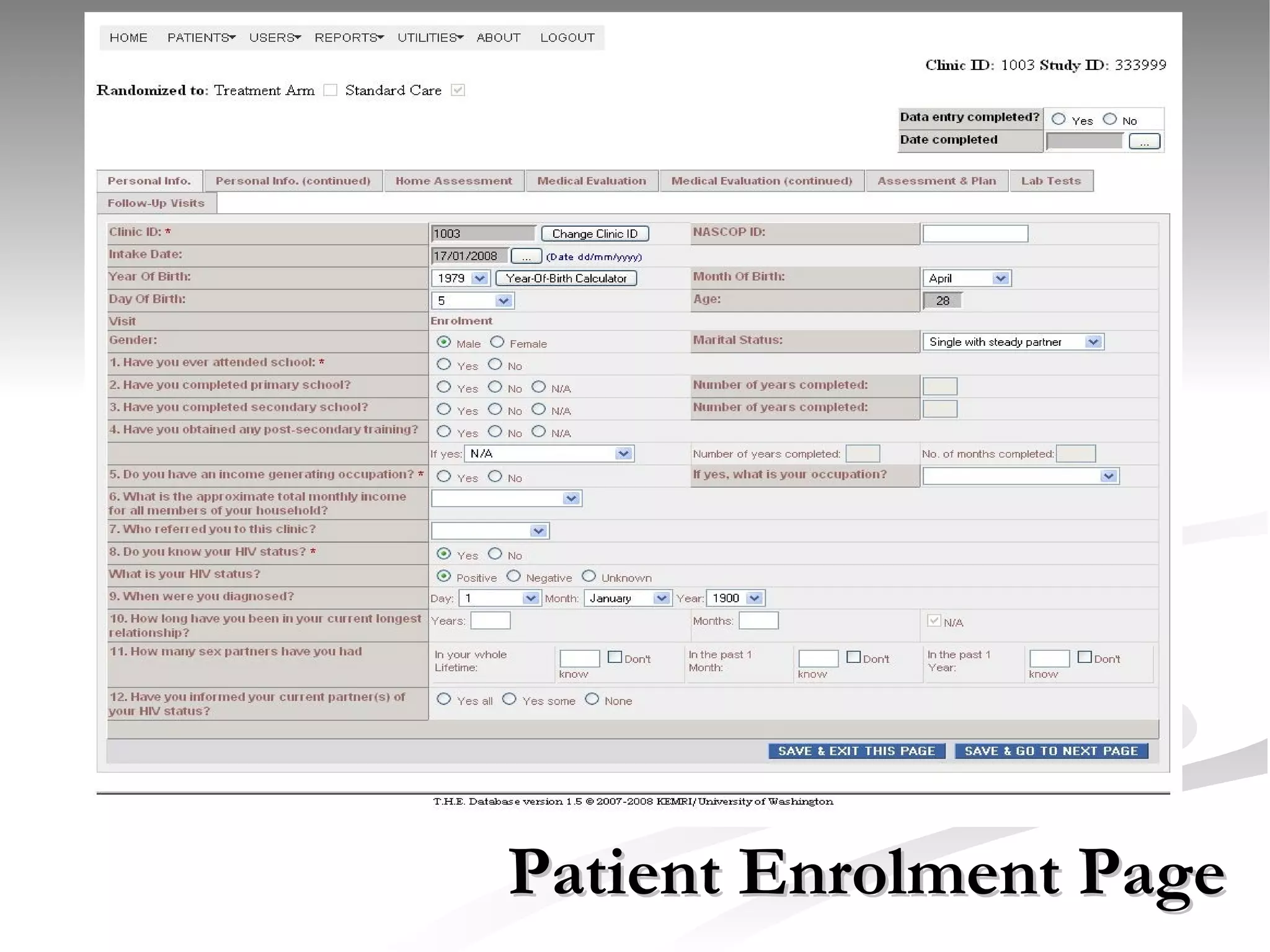
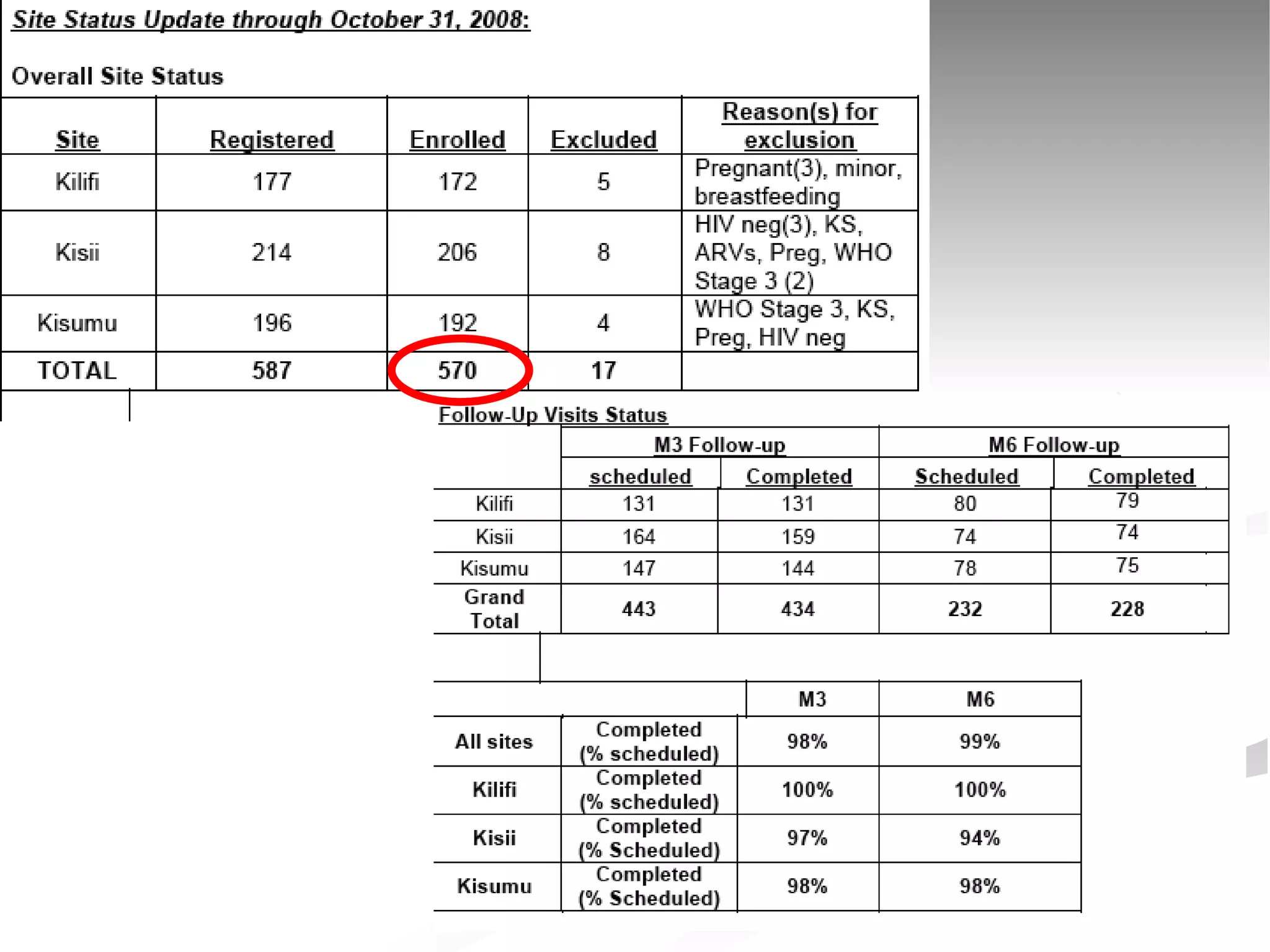
The document discusses delaying HIV-1 disease progression in individuals with co-infections, particularly helminths, in resource-limited settings. It highlights the prevalence of helminth co-infection among HIV-1 positive individuals in Africa and outlines a study aiming to evaluate the effects of treating helminth infections on HIV-1 disease markers. The study's objectives include assessing the impact of deworming therapies on CD4 counts and viral load in HIV-1 positive adults, while also addressing operational challenges and future research directions.