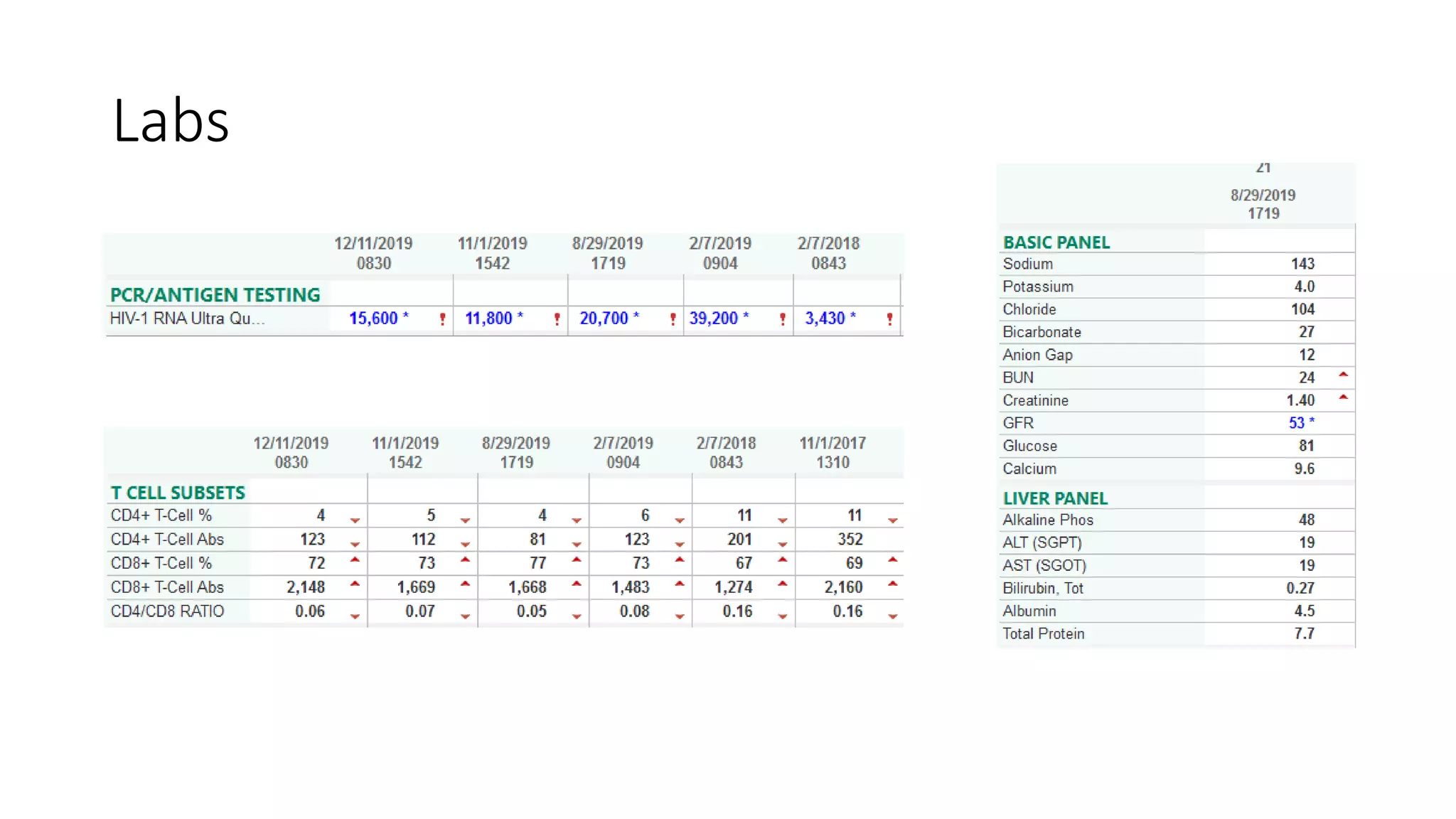
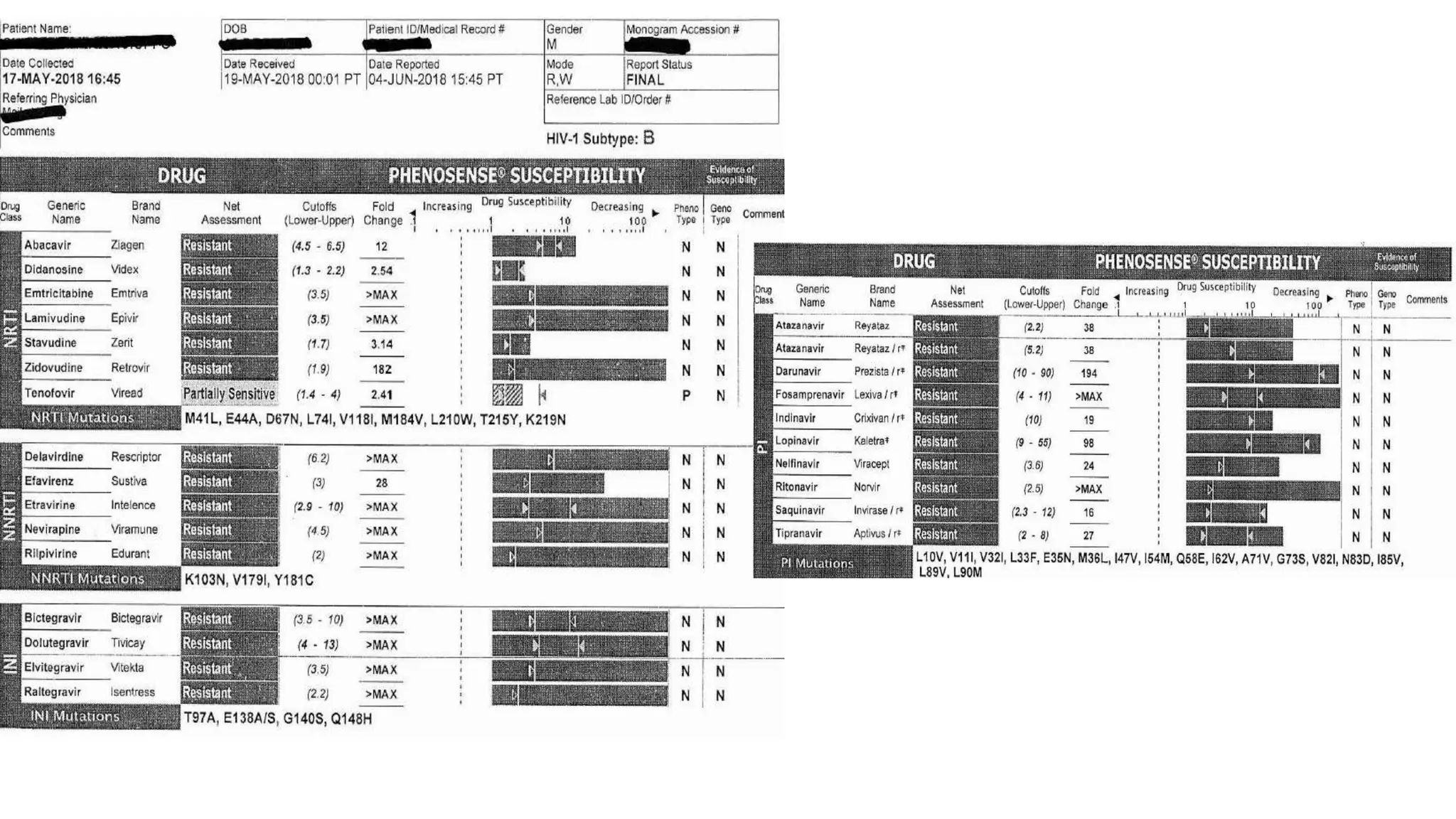
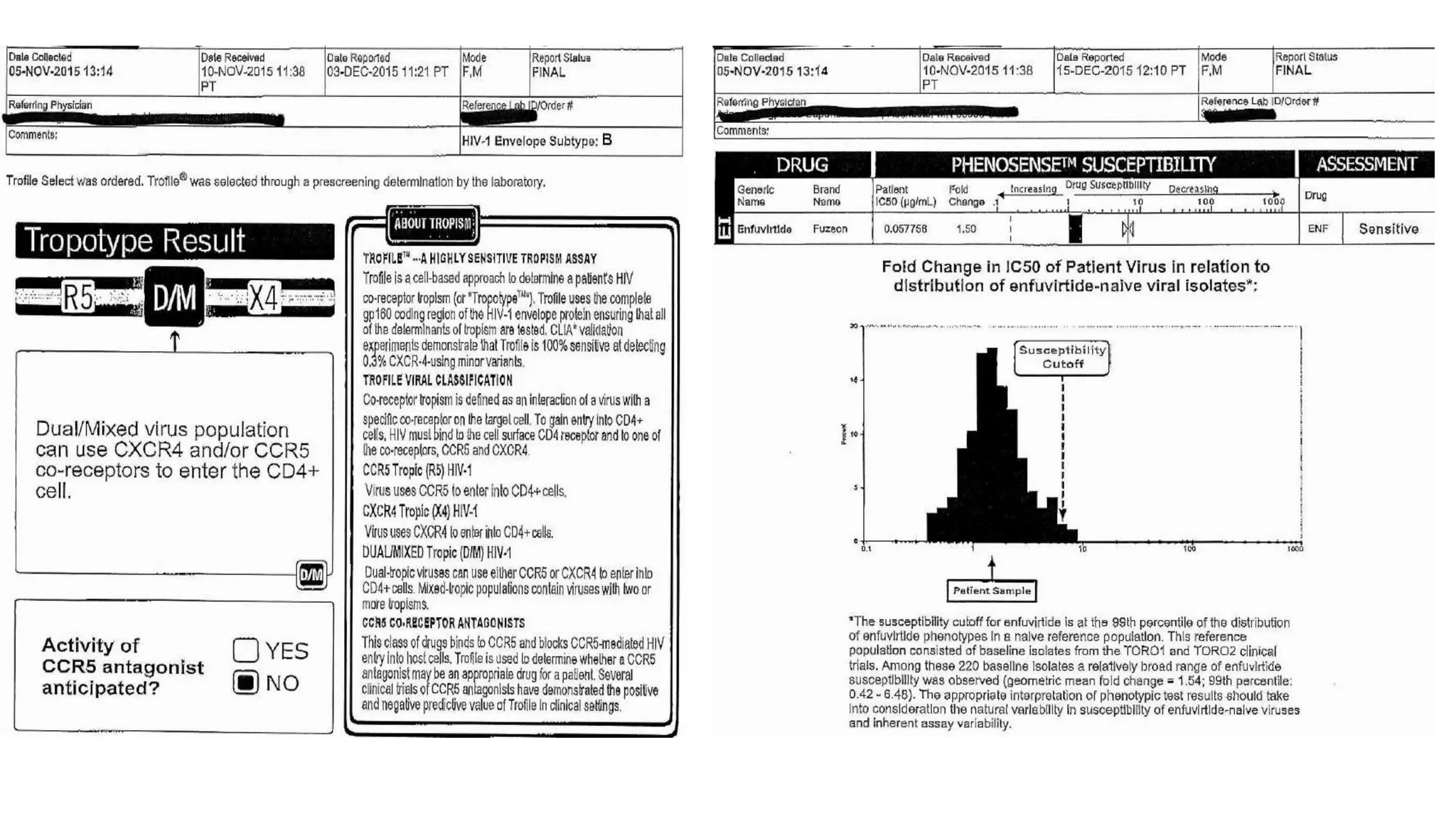
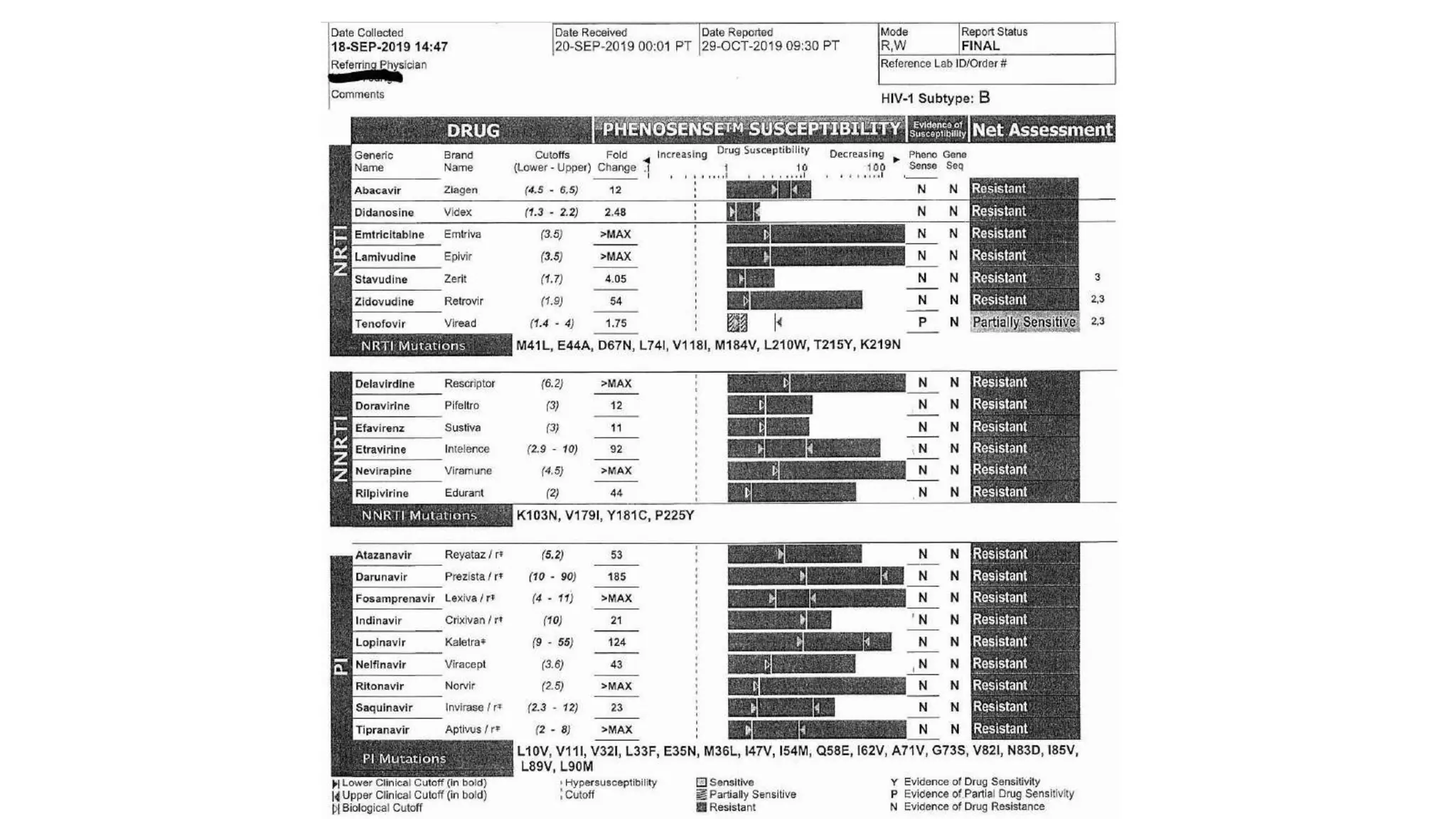
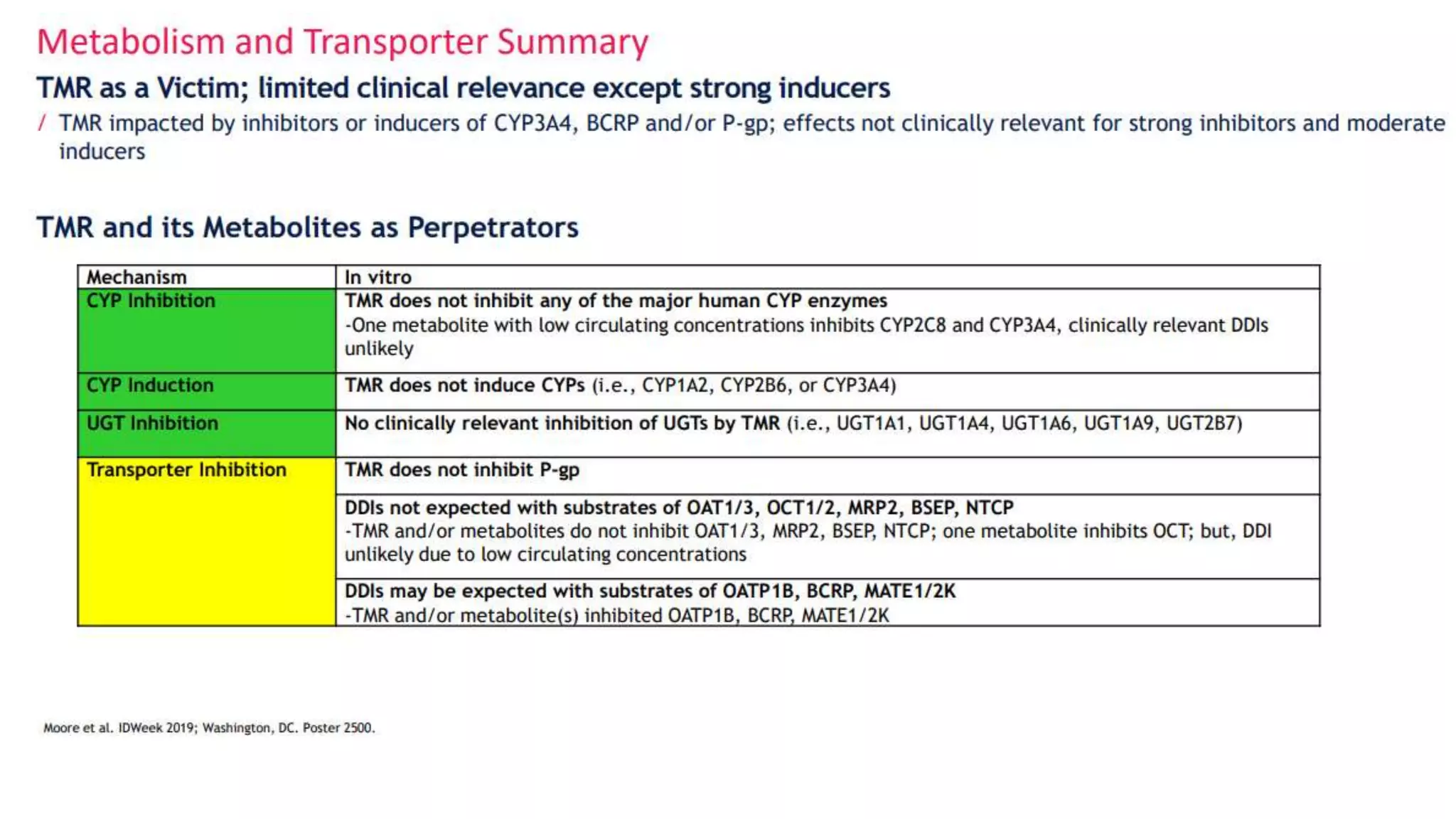
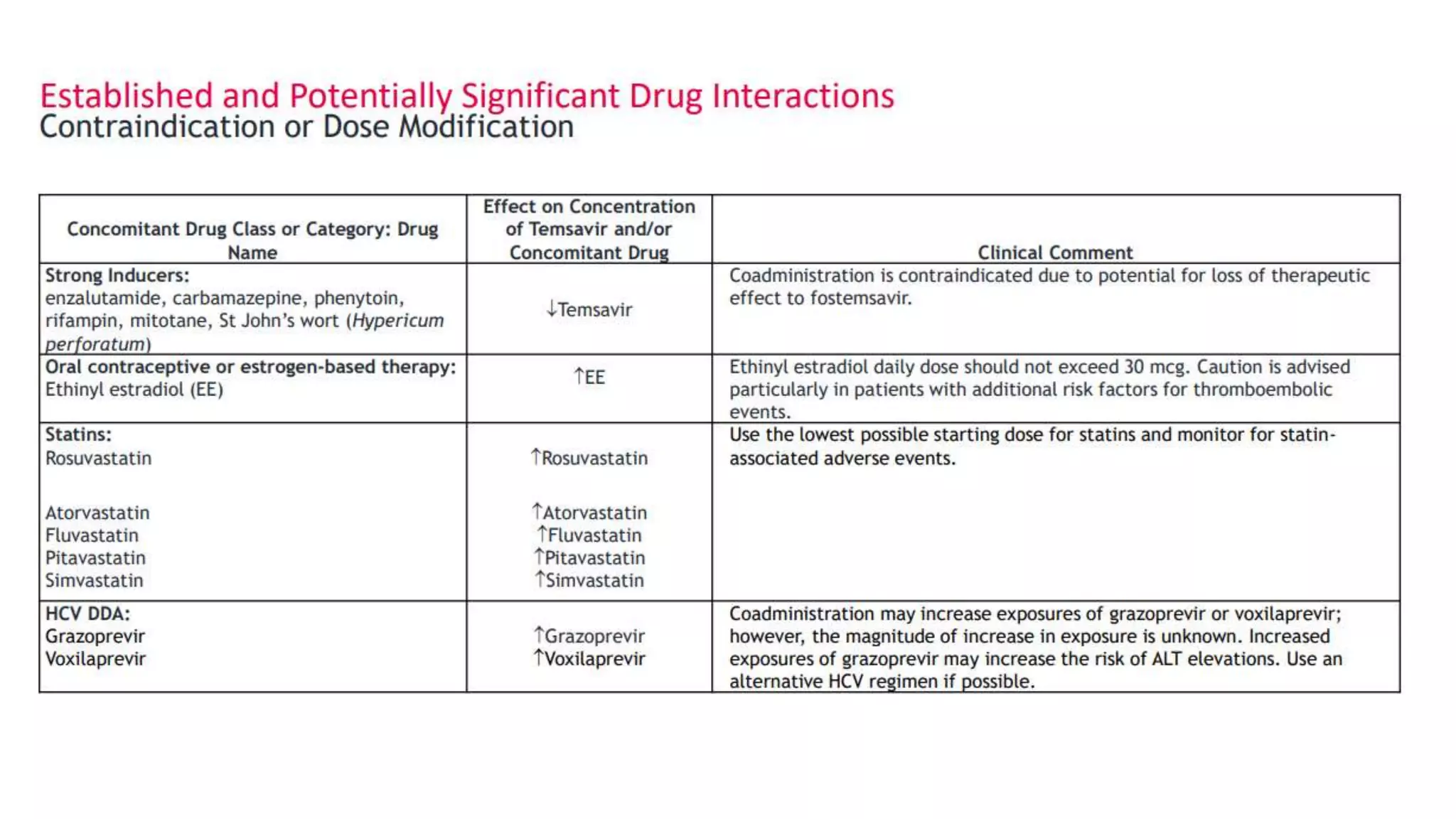
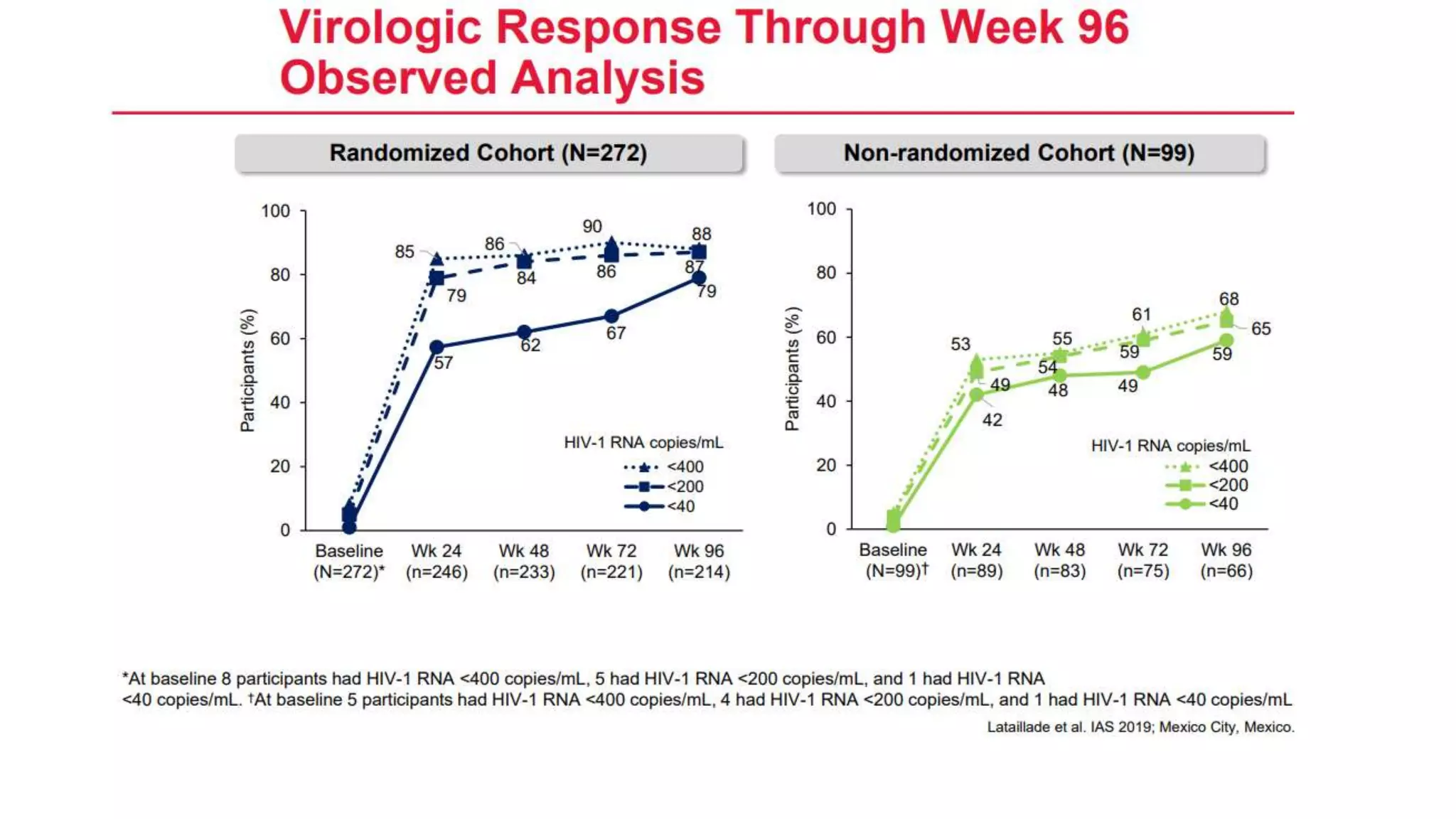
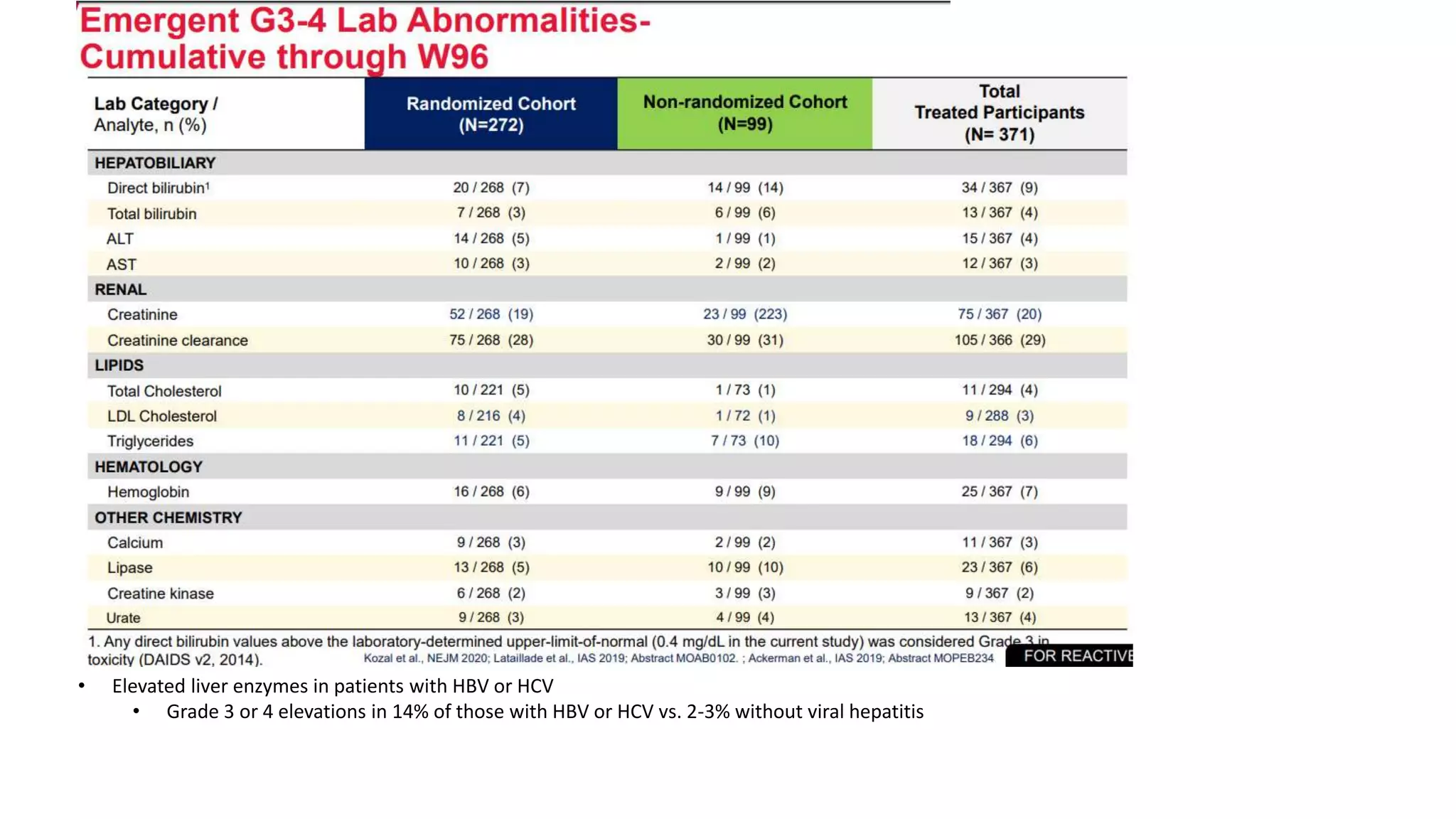
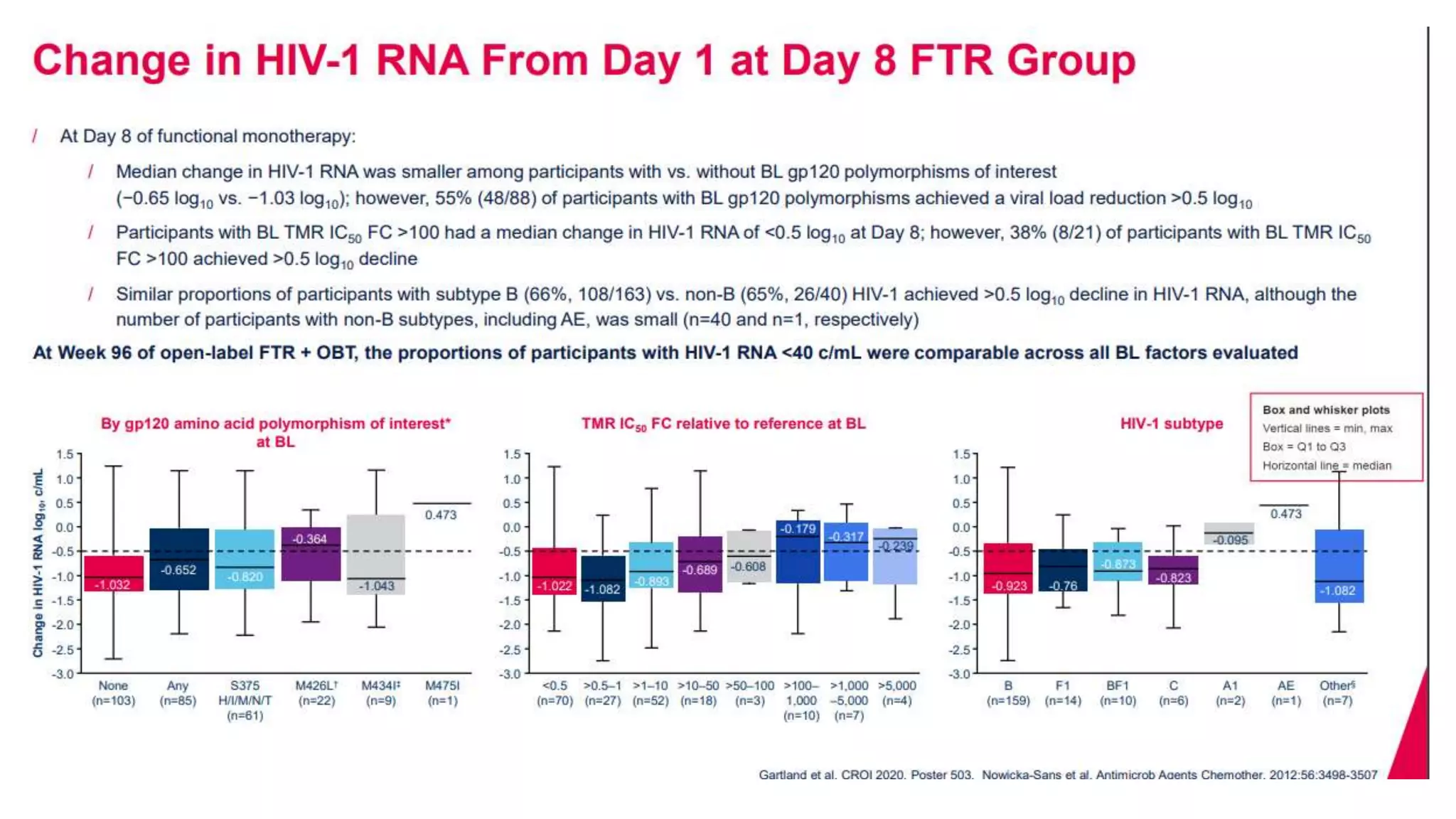
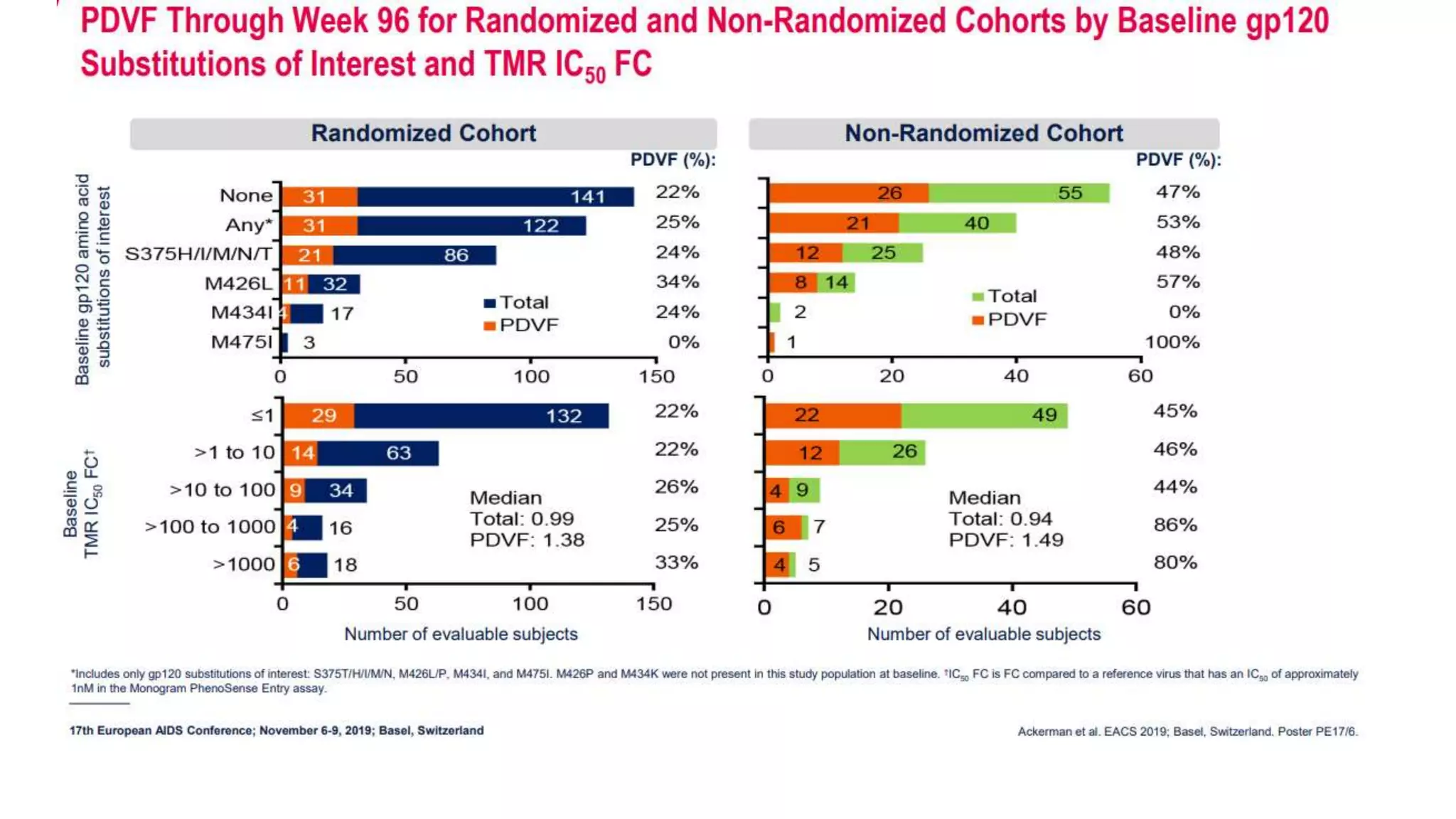
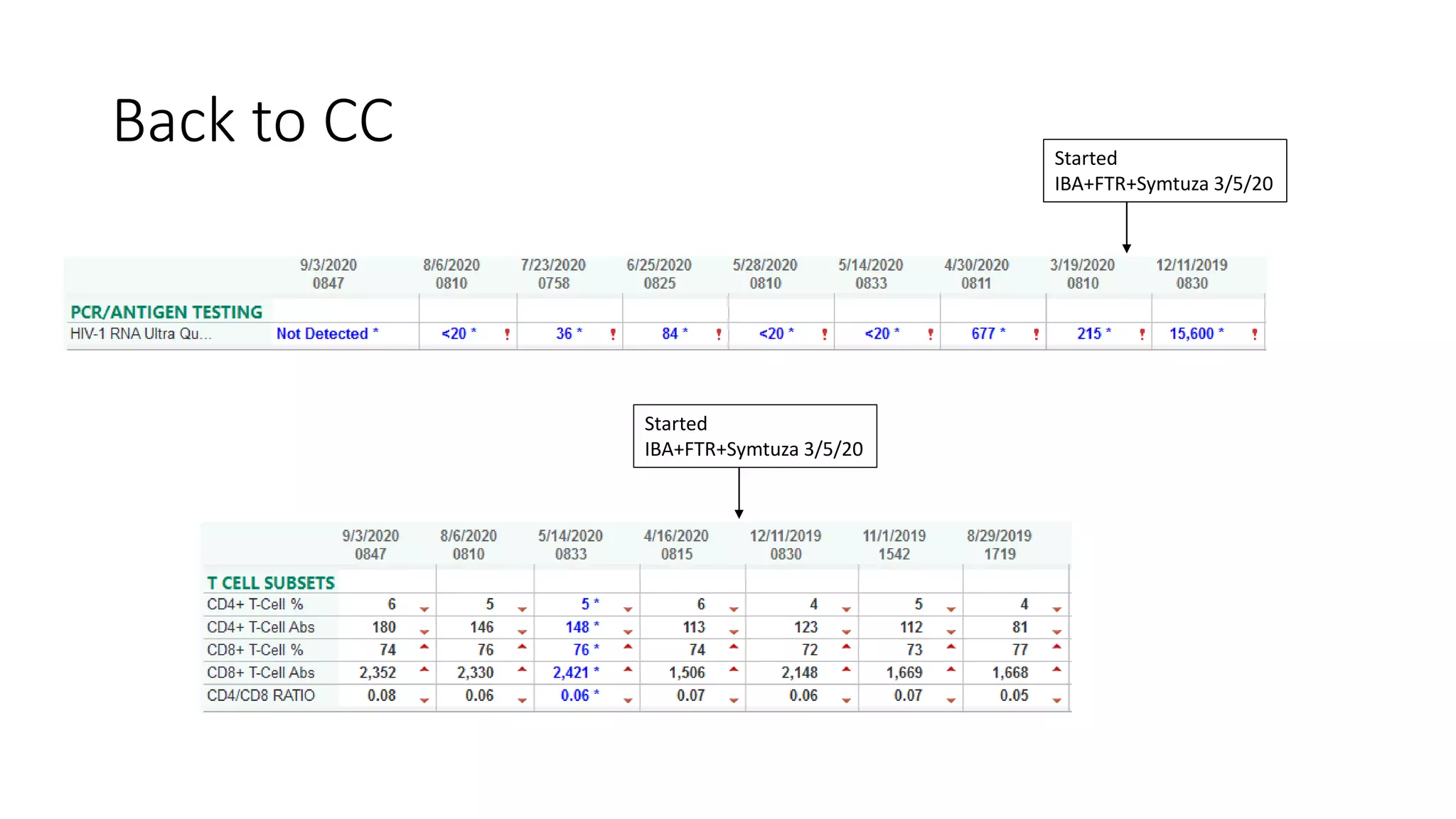
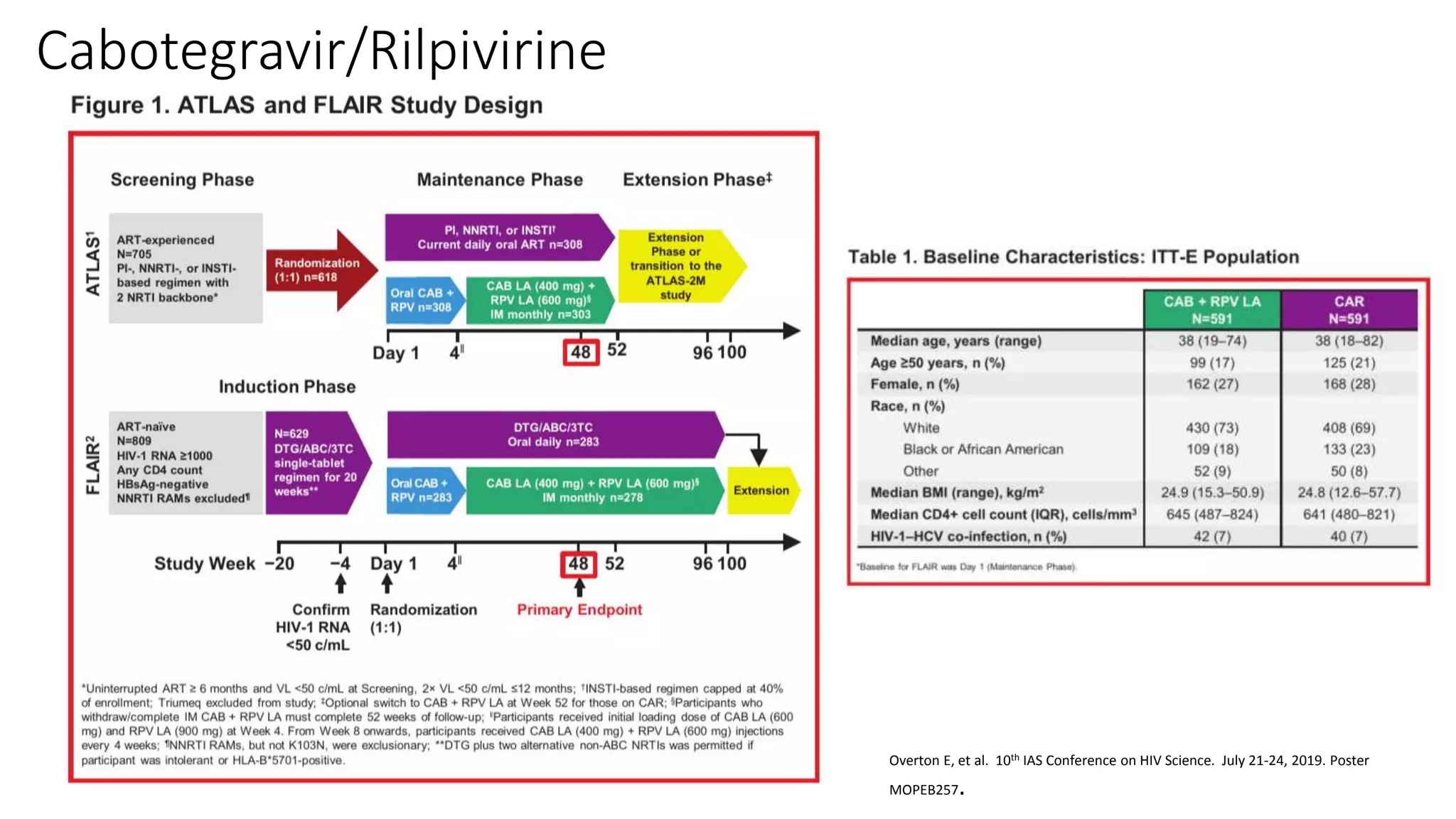
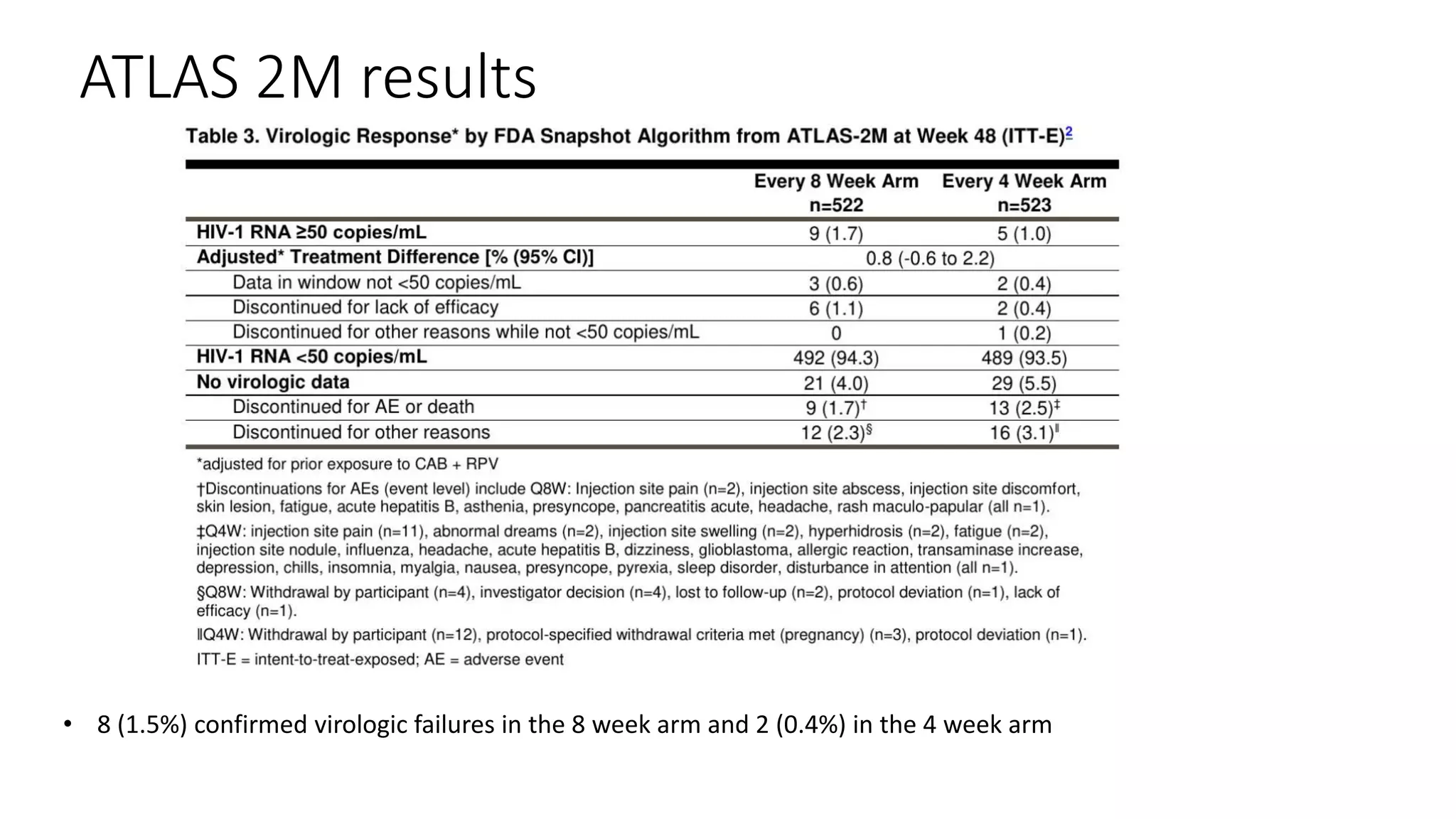
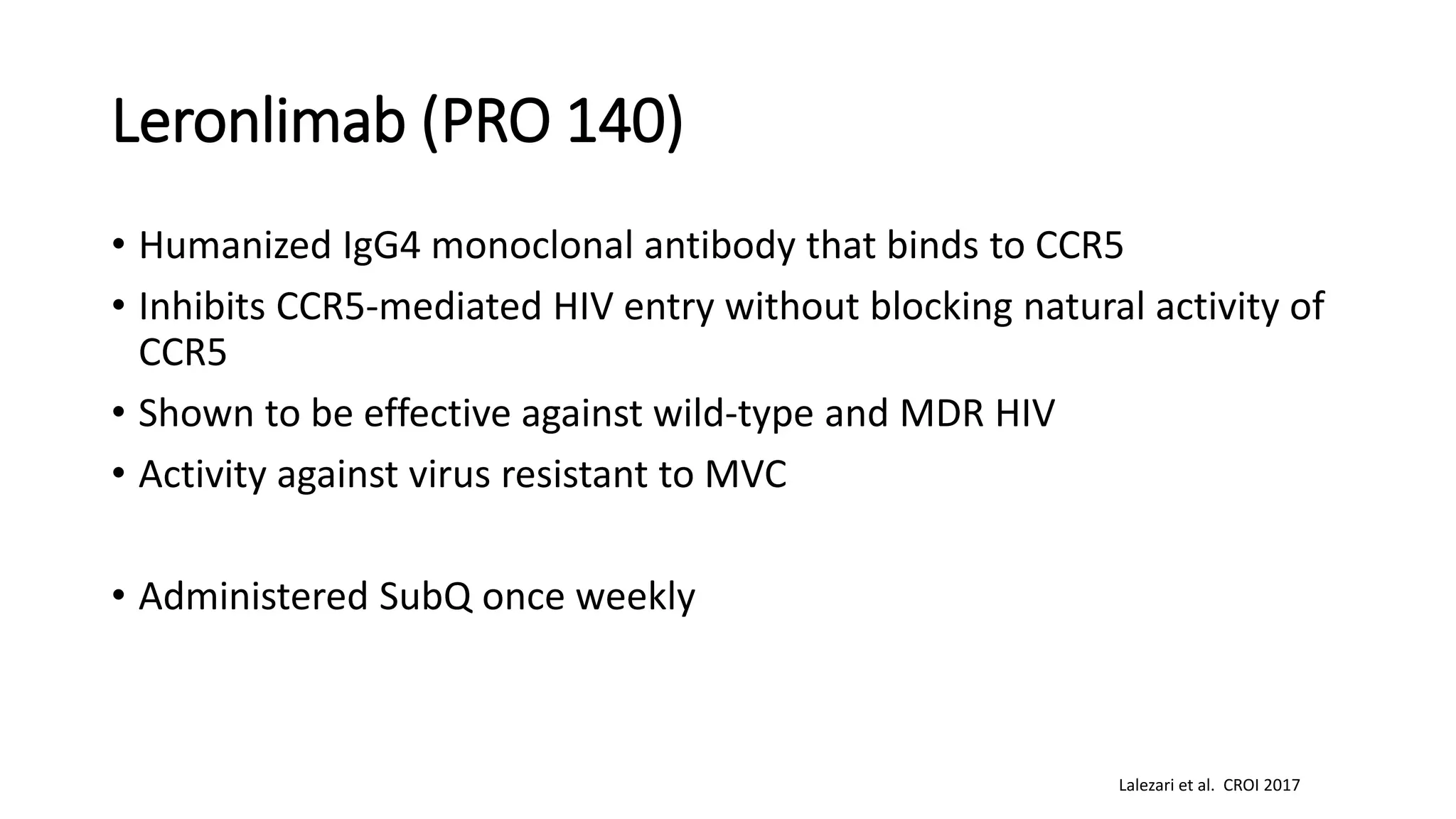
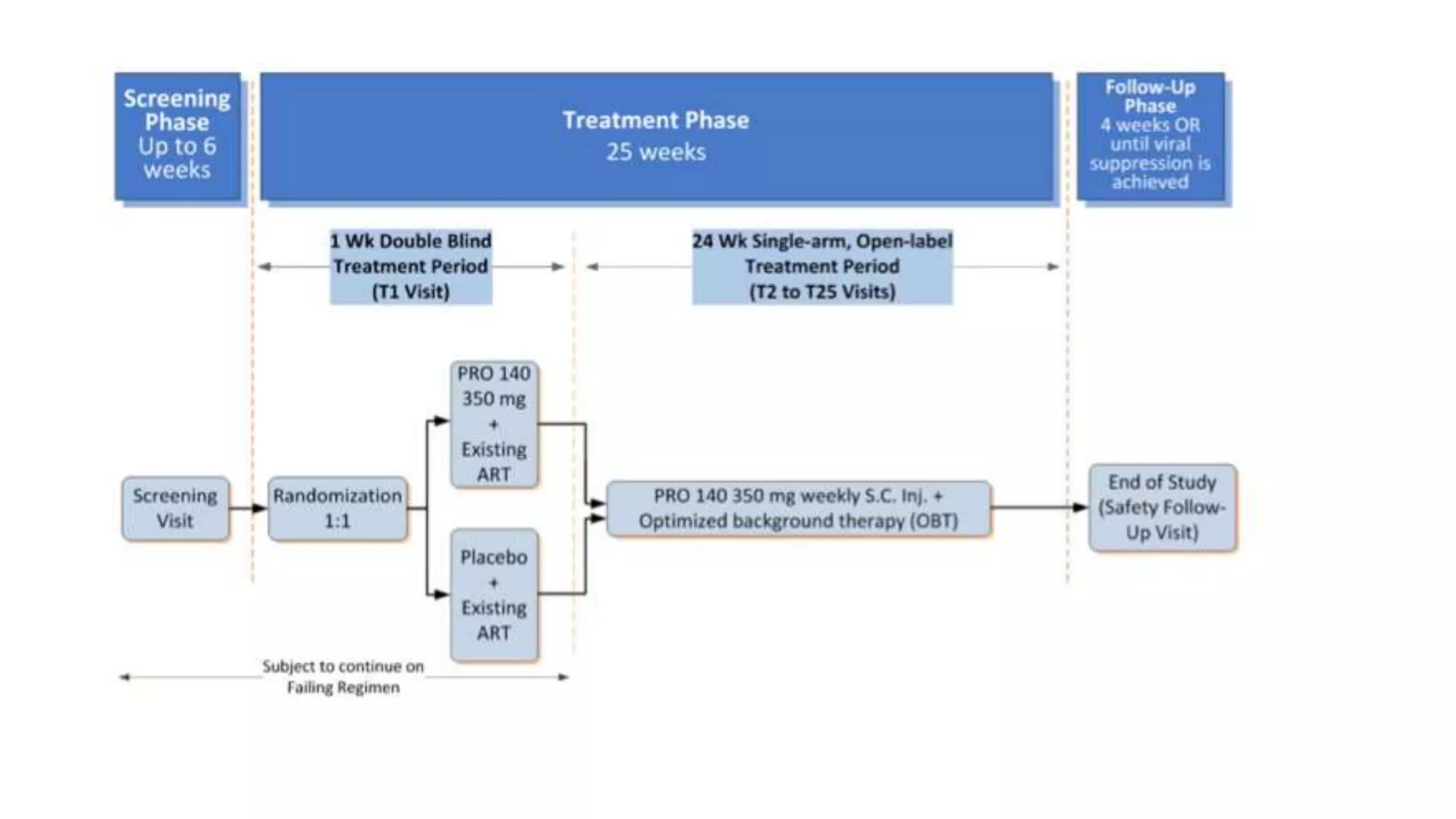
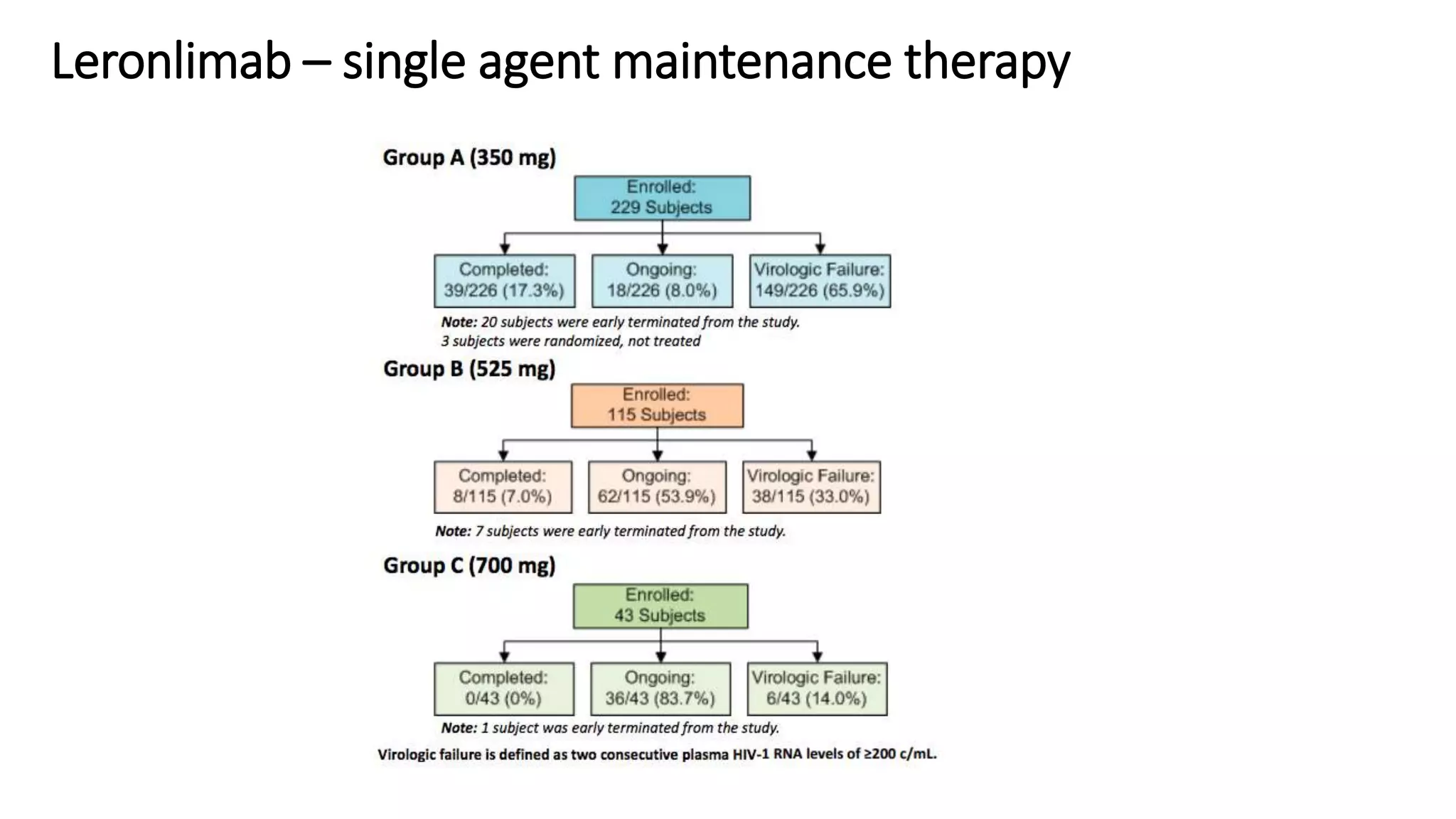
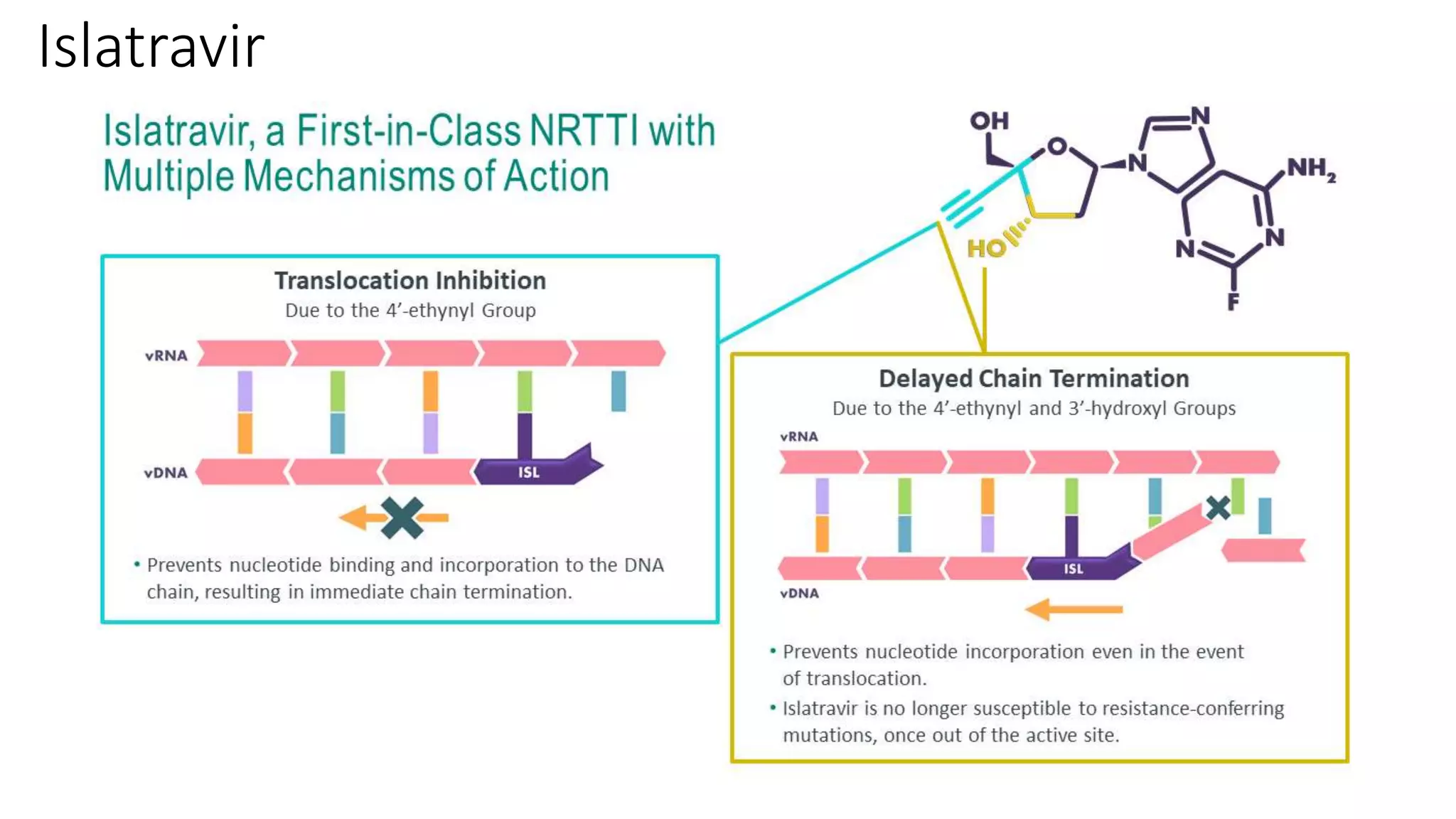
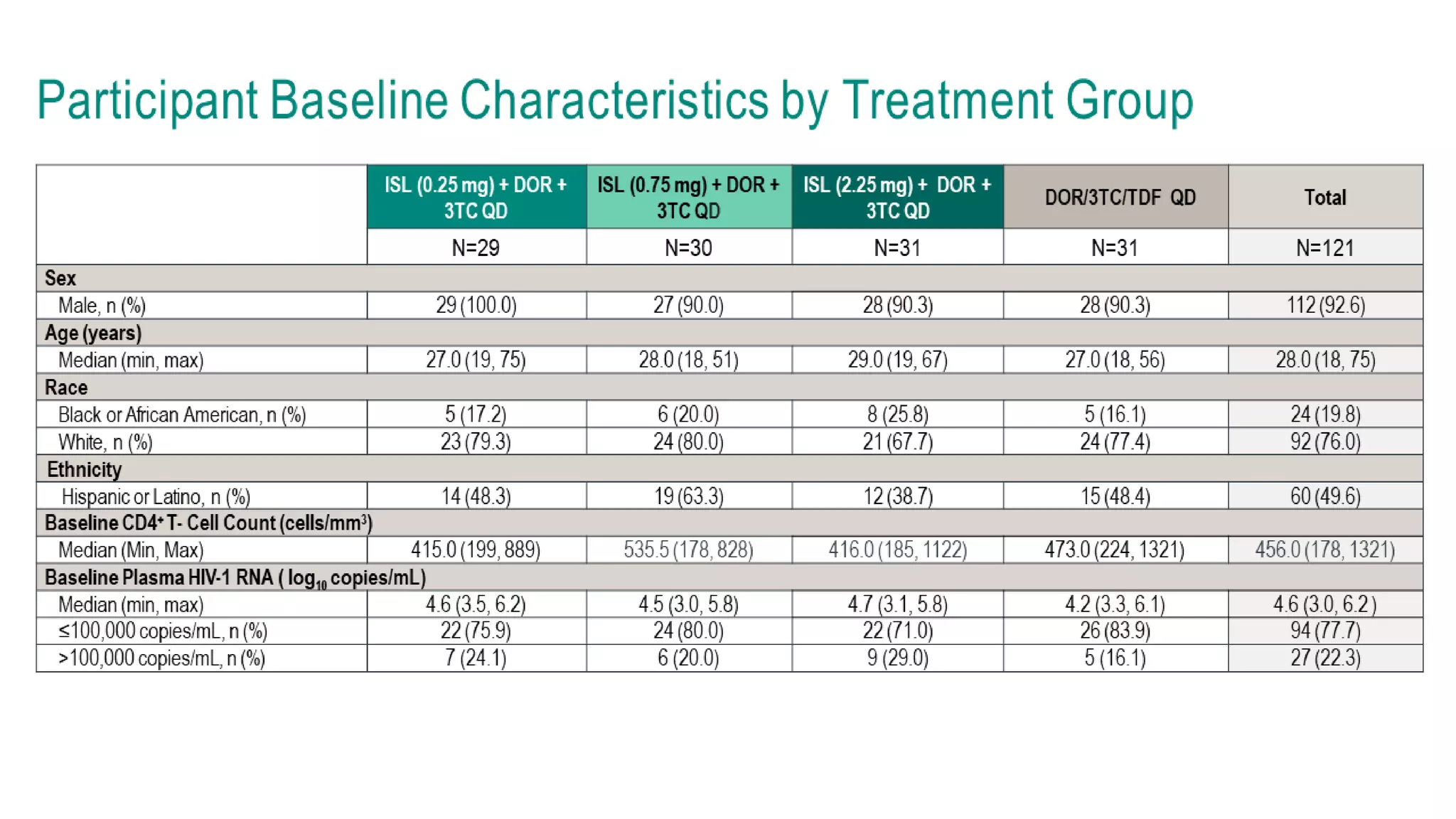
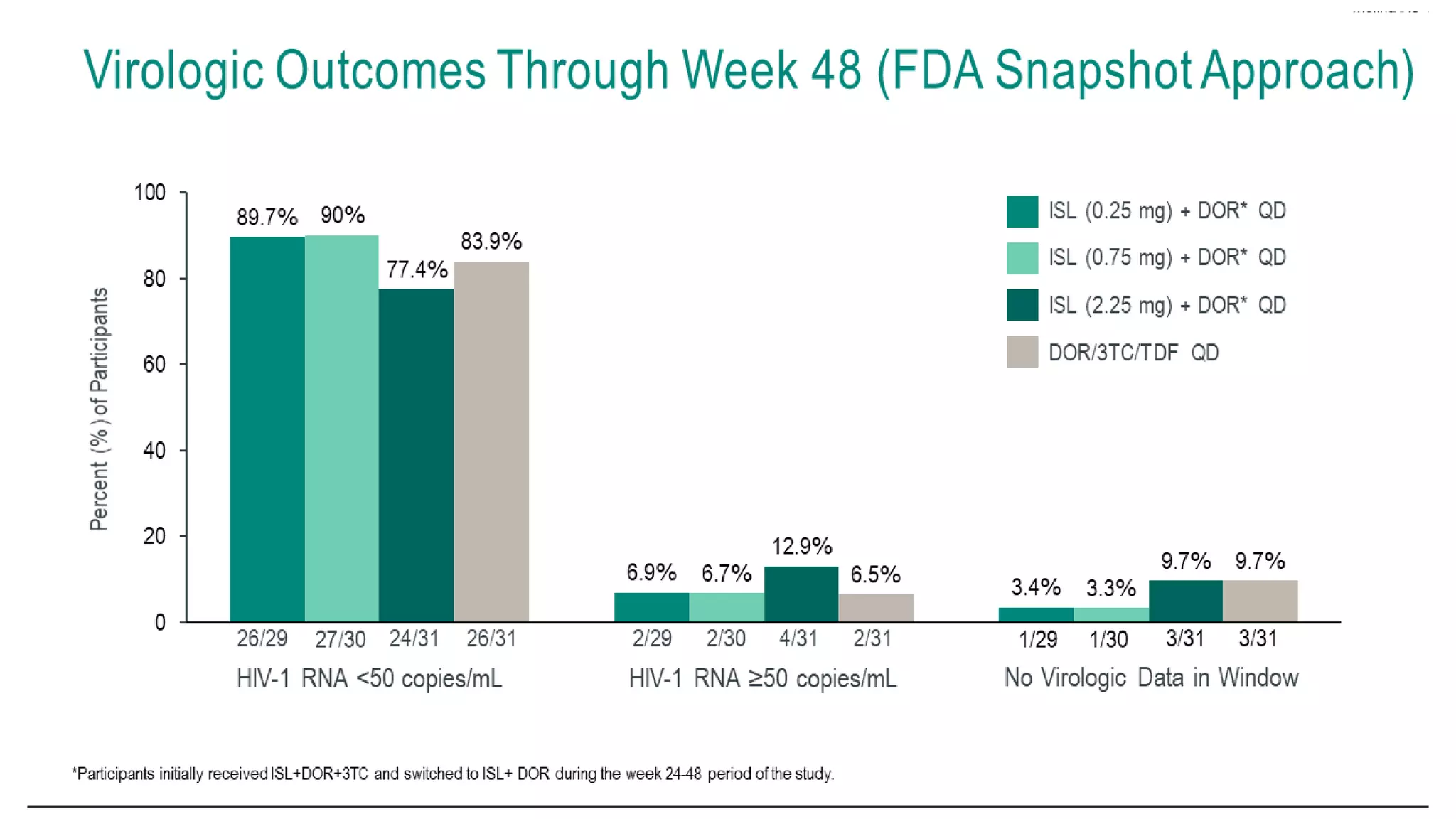
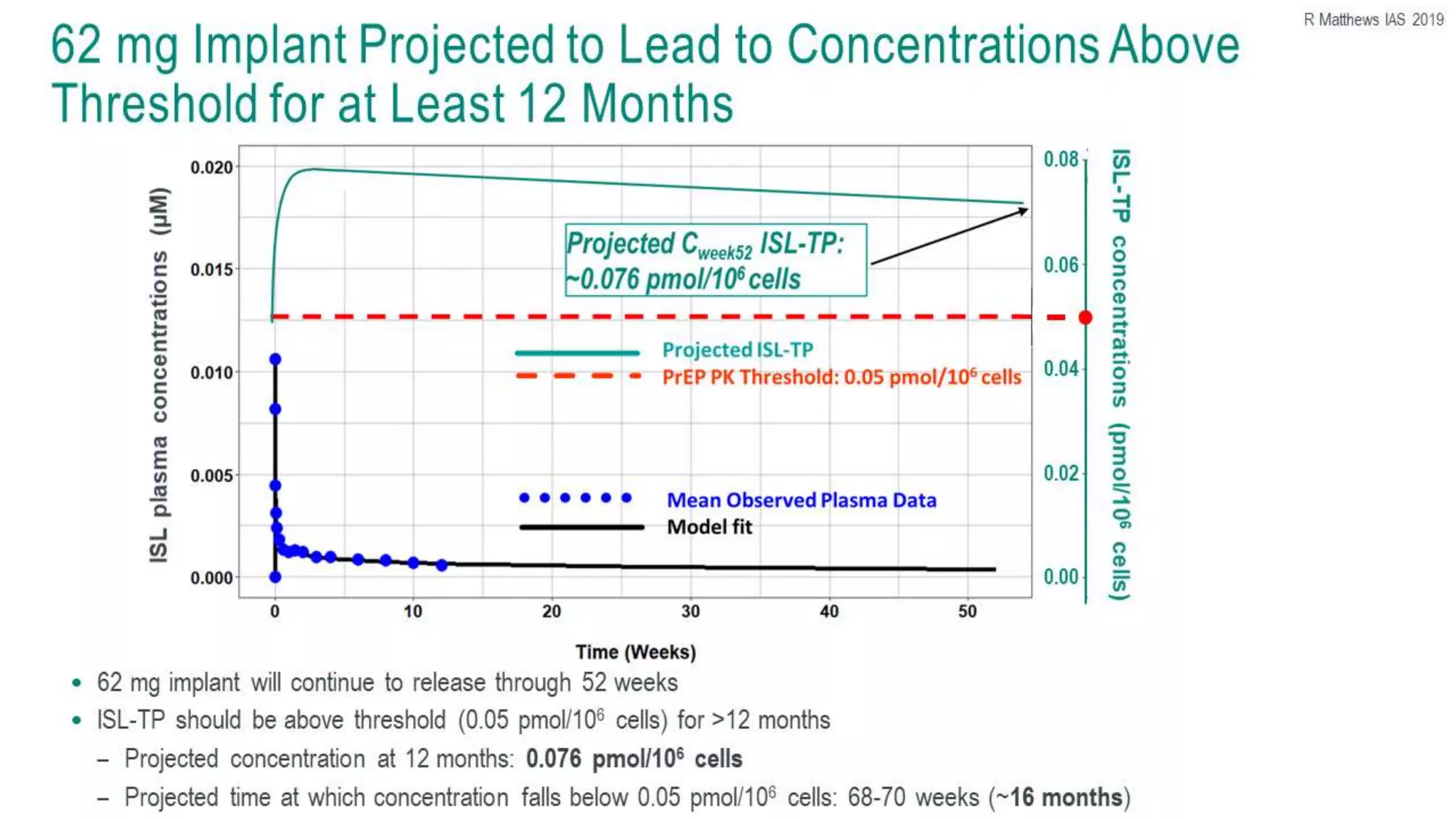
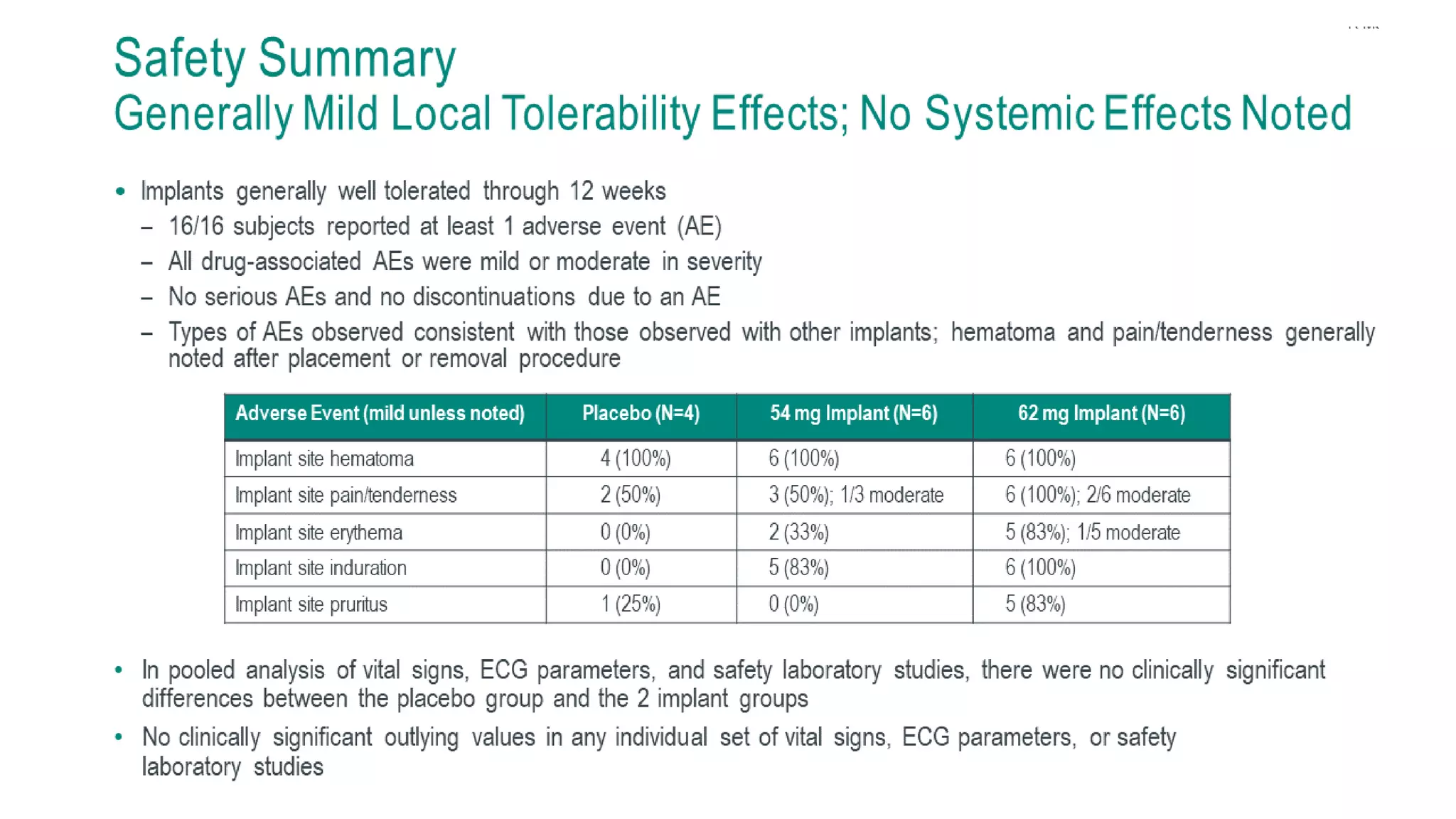
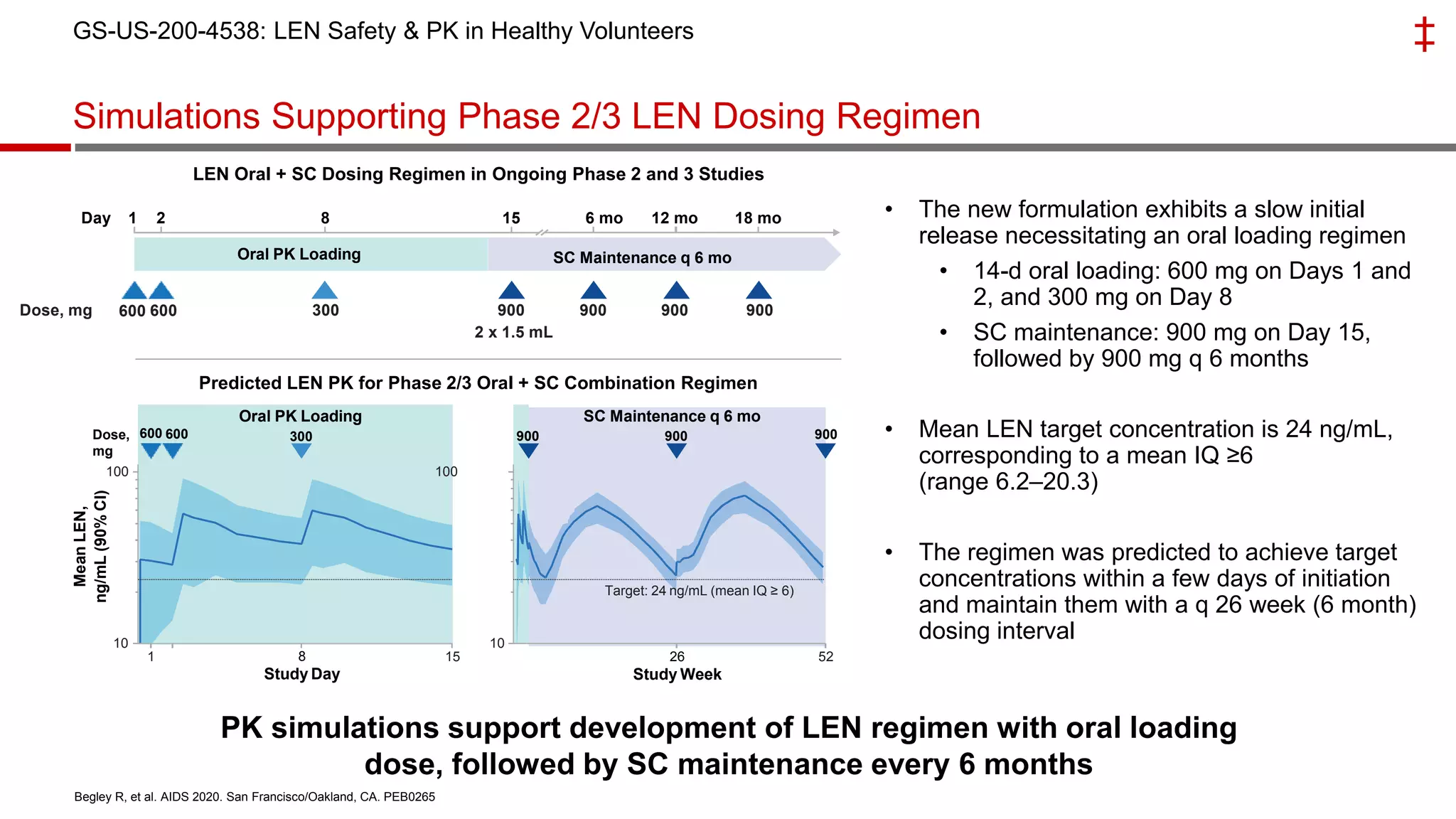
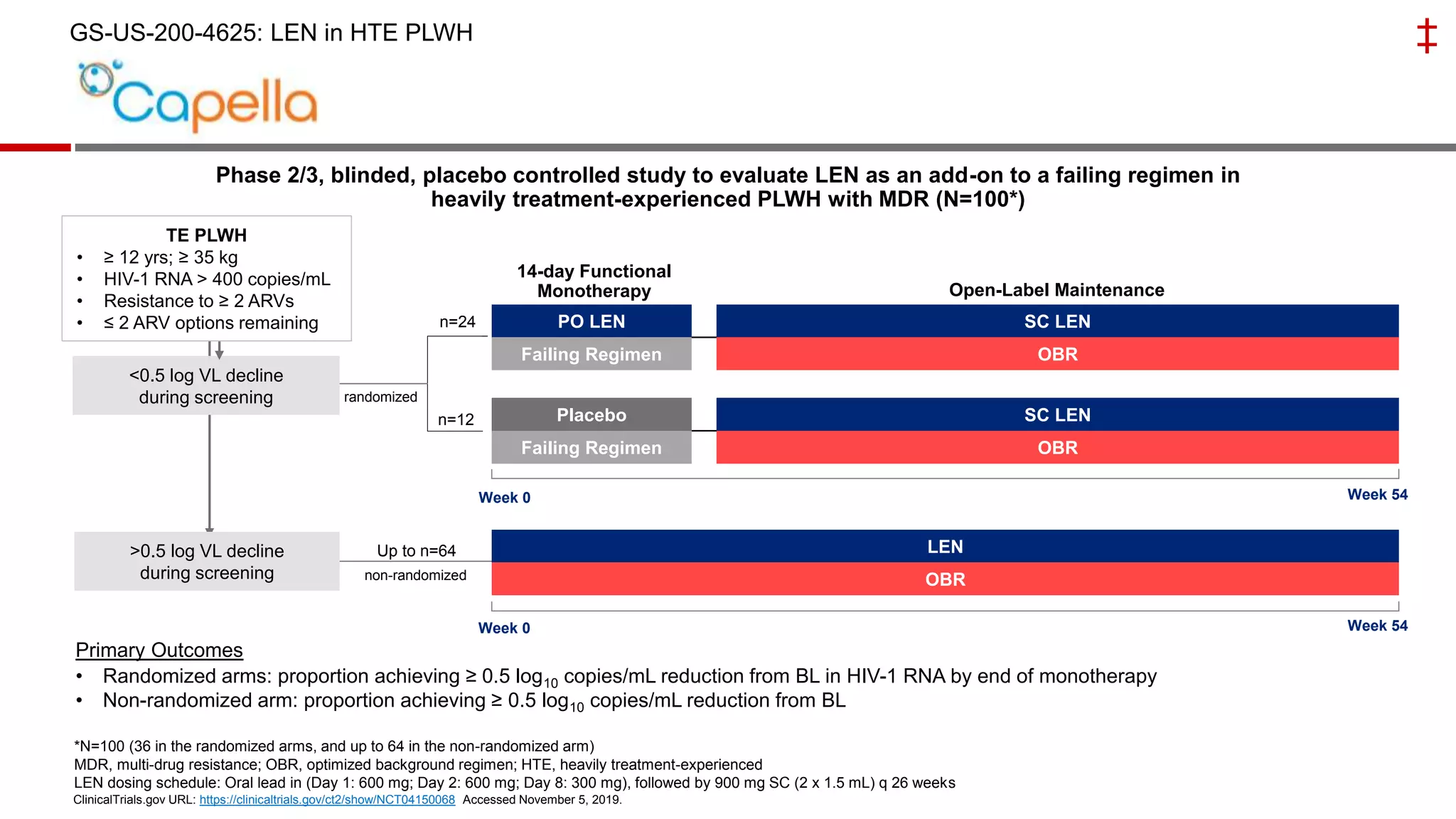
This document summarizes a presentation on new and investigational antiretrovirals given at the UC San Diego HIV & Global Health Rounds. The presentation reviewed fostemsavir, cabotegravir/rilpivirine, leronlimab, islatravir, and lenacapavir. For each drug, the presenter discussed indications, dosing, efficacy and safety data from clinical trials, resistance profiles, and potential advantages and limitations. The goal of the HIV & Global Health Rounds is to provide clinicians and researchers with the most up-to-date information on HIV, hepatitis, tuberculosis, and other infectious diseases.