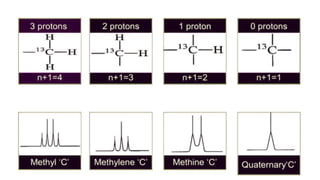
Deuterium coupling involves the replacement of hydrogen with deuterium in molecules, affecting NMR spectroscopy signals. This phenomenon includes intramolecular and intermolecular coupling, with the latter commonly observed in deuterated solvents. Off-resonance decoupling in C-13 NMR allows for reduced multiplicity of carbon signals, enhancing structural information by indicating attached hydrogens.