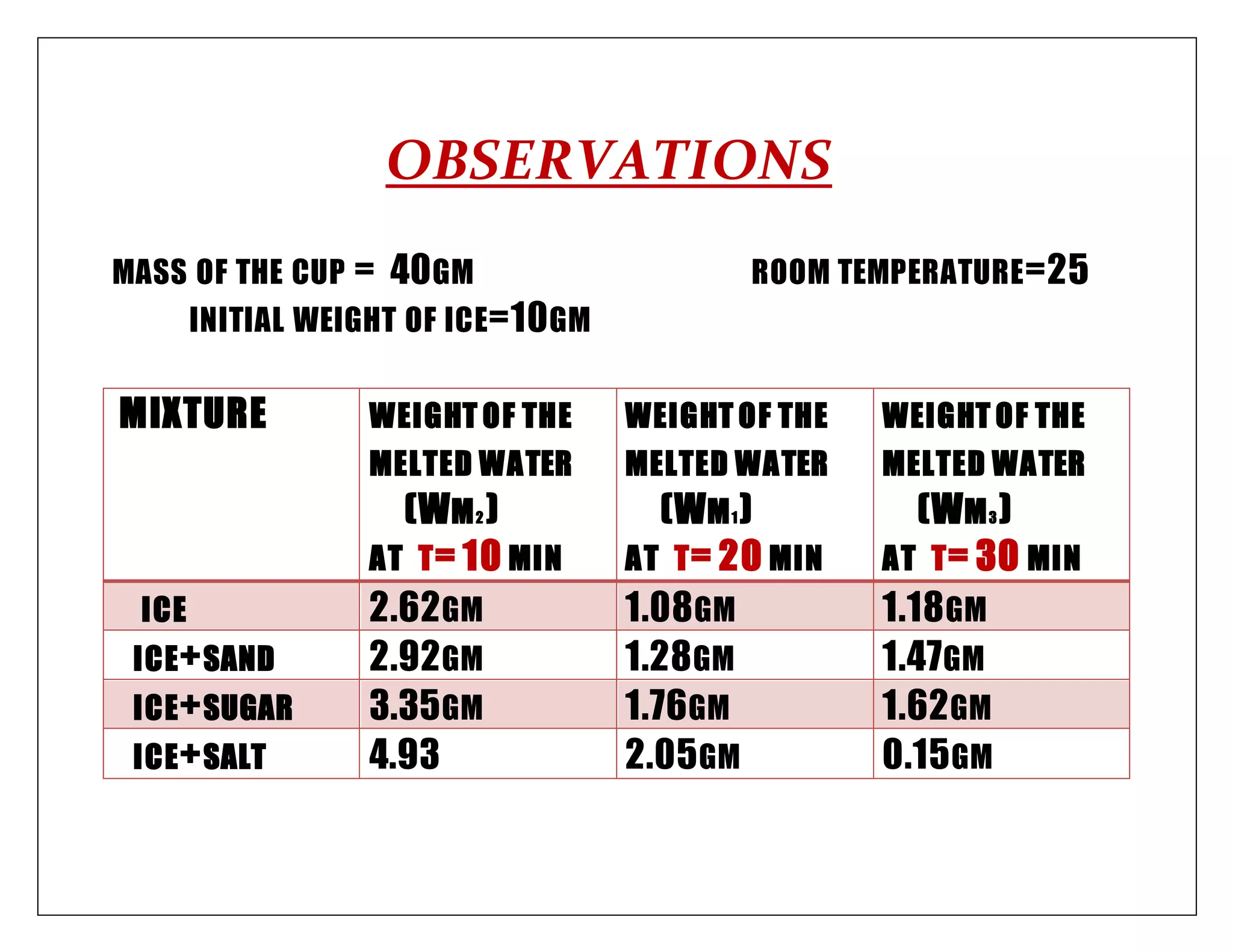
This document appears to be a chemistry project report submitted by a student. It investigates which substance (salt, sugar, or sand) causes ice cubes to melt the fastest when added. The procedure involves measuring the mass of melted water over time for ice cubes with each substance added. Calculations determine the percentage of ice melted for each substance. The results show that salt causes the greatest increase in melting percentage, followed by sugar then sand, indicating salt causes the greatest depression of the freezing point.
![2014-2015
Class XII Sci C
Vrinda Singh
[CHEMISTRY PROJECT]
Delhi Public School, Jaipur](https://image.slidesharecdn.com/finalchemistryproject-150131222424-conversion-gate02/75/depression-in-freezing-point-1-2048.jpg)









![5. REPEAT THREE TIMES FOR EACH SUBSTANCE TO BE TESTED.
6. USE THE SAME PROCEDURE TO MEASURE THE MELTING RATE FOR ICE
CUBES WITH NOTHING ADDED.
7.FOR EACH TEST, CALCULATE THE PERCENTAGE OF THE ICE CUBE THAT
MELTED:
[MASS OF MELTED WATER]/[INITIAL MASS OF CUBE] X 100
8. FOR EACH TEST, CALCULATE THE PERCENTAGE OF ICE CUBE
REMAINING:
[REMAINING MASS OF ICE CUBE]/ [INITIAL MASS OF ICE CUBE] X 100
9. FOR EACH SUBSTANCE YOU TESTED, CALCULATE THE AVERAGE
AMOUNT OF MELTED WATER PRODUCED (AS A PERCENT OF INITIAL](https://image.slidesharecdn.com/finalchemistryproject-150131222424-conversion-gate02/75/depression-in-freezing-point-12-2048.jpg)


![CALCULTIONS
% OF MELTING= [MASS OF MELT WATER] X 100
[INITIAL MASS OF ICE CUBE]
% OF (ICE+SALT MELTED)1 = WM1 X 100= 49.3%
WI
% OF (ICE+SALT MELTED)2 = WM2 X 100= 20.5%
WI
% OF (ICE+SALT MELTED)3 = WM3 X 100= 1.5%
WI
AVERAGE %OF (ICE+SALT MELTED) = %1+%2+%3 =23.78%](https://image.slidesharecdn.com/finalchemistryproject-150131222424-conversion-gate02/75/depression-in-freezing-point-16-2048.jpg)
![CALCULTIONS
% OF MELTING= [MASS OF MELT WATER] X 100
[INITIAL MASS OF ICE CUBE]
% OF (ICE+SAND MELTED)1 = WM1 X 100=29.2%
WI
% OF (ICE+SAND MELTED)2 = WM2 X 100= 12.8%
WI
% OF (ICE+SAND MELTED)3 = WM3 X 100=14.8%
WI
AVERAGE %OF (ICE+SAND MELTED) = %1+%2+%3 =18.9%
3](https://image.slidesharecdn.com/finalchemistryproject-150131222424-conversion-gate02/75/depression-in-freezing-point-18-2048.jpg)
![CALCULTIONS
% OF MELTING= [MASS OF MELT WATER] X 100
[INITIAL MASS OF ICE CUBE]
% OF (ICE+SUGAR MELTED)1 = WM1 X 100=33.5%
WI
% OF (ICE+SUGAR MELTED)2 = WM2 X 100=17.6%
WI
% OF (ICE+SUGAR MELTED)3 = WM3 X 100=16.2%
WI](https://image.slidesharecdn.com/finalchemistryproject-150131222424-conversion-gate02/75/depression-in-freezing-point-19-2048.jpg)




