1) The document discusses creating a chemical compound by making homemade ice cream.
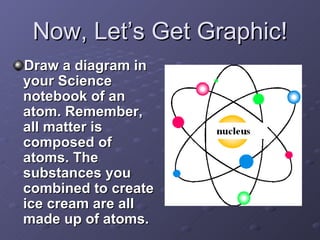
2) A chemical compound is formed when two or more elements are chemically joined together, unlike a mixture where substances are mixed but not joined.
3) To make the ice cream, students combine milk, sugar, creamer and vanilla in a bag, then shake the bag inside a separate bag filled with ice and salt to cause the ingredients to freeze into a compound of ice cream.















!["Ice Cream Chemistry" Real Cool Science Designed and created by: Ms. V. Mitchell Using SmartBoard Technology [email_address] Public Domain](https://image.slidesharecdn.com/icecreamchemistry-13117819764809-phpapp02-110727105421-phpapp02/85/Ice-Cream-Chemistry-17-320.jpg)