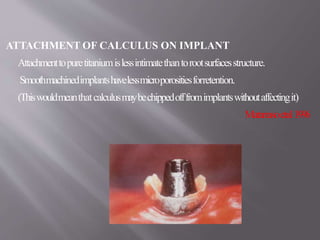
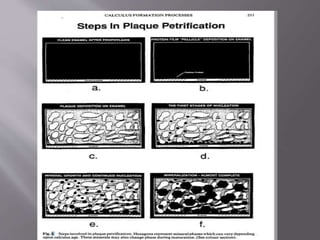
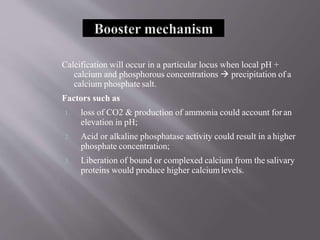
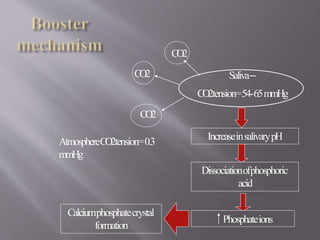
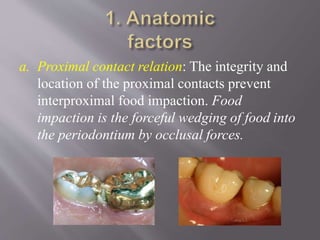
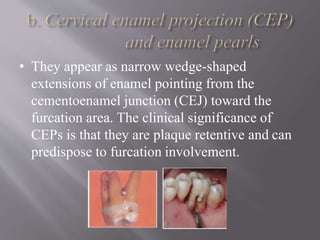
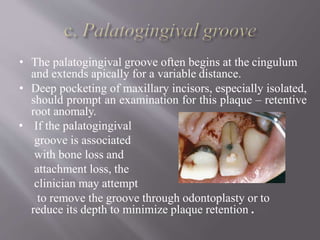
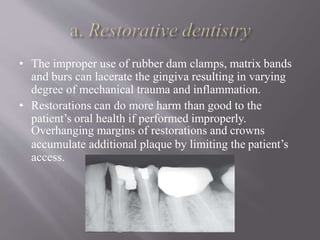
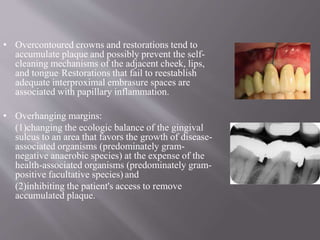
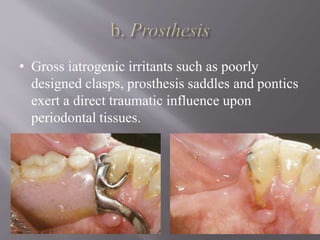
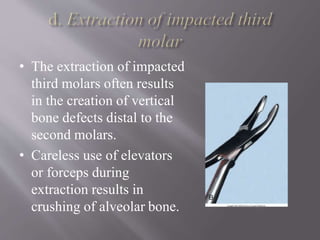
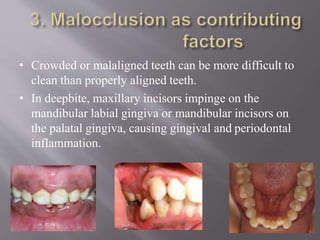
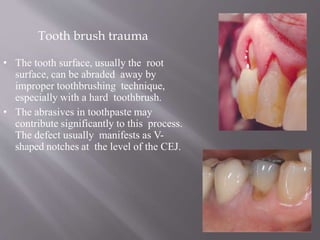
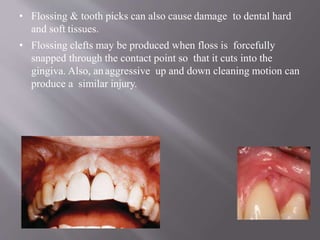
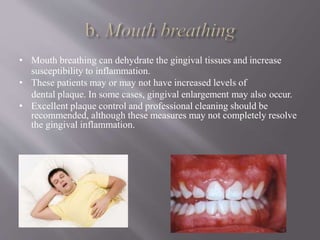
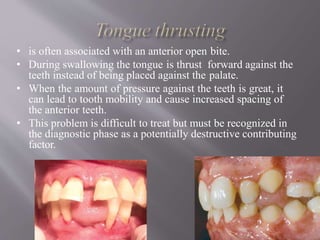
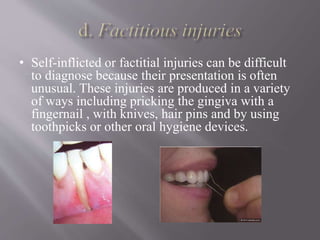
This document discusses dental calculus, including its definition, classification, prevalence, formation, and clinical significance. It covers several key points:
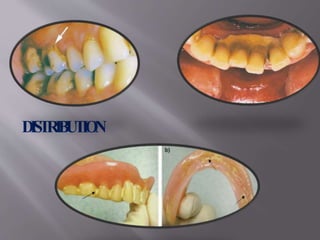
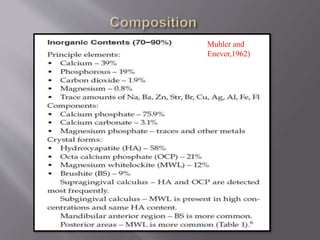
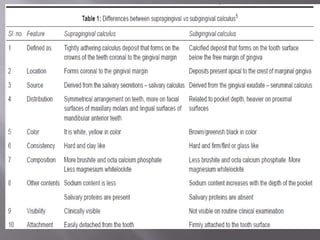
- Calculus is mineralized dental plaque that forms on tooth surfaces. It consists primarily of hydroxyapatite and other calcium phosphate minerals.
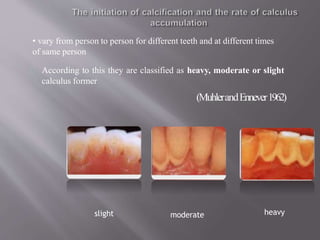
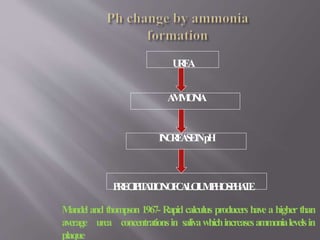
- Calculus formation starts within days as plaque mineralizes. By age 30, most people have calculus covering all tooth surfaces.
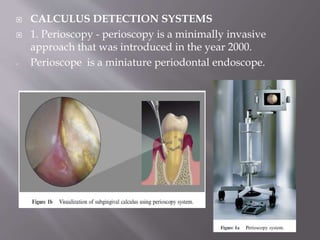
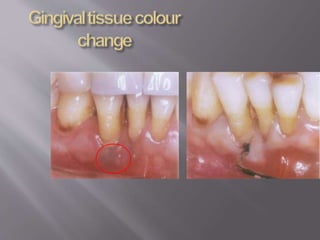
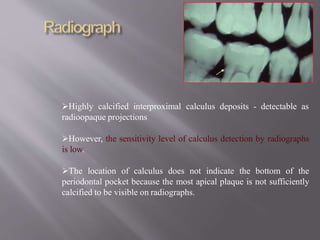
- Calculus promotes further plaque buildup and can harbor bacteria. It is detected using visual, tactile, radiographic, and newer optical methods.
- Removing calculus through scaling disrupts the bacterial matrix and is an important part of periodontal therapy. Various chemical and enzymatic