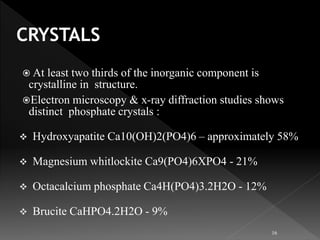
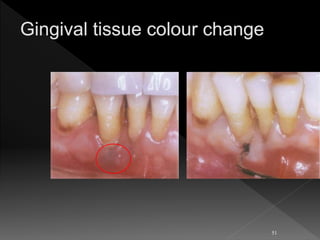
This document presents a comprehensive overview of dental calculus, detailing its definitions, historical perspectives, components, microbiology, and methods of formation and attachment. It highlights the shift in understanding from calculus as the primary cause of periodontal disease to the significance of dental plaque, along with the classification and characteristics of both supragingival and subgingival calculus. Furthermore, it discusses various theories regarding the mineralization process and the relationship between calculus and oral microorganisms.