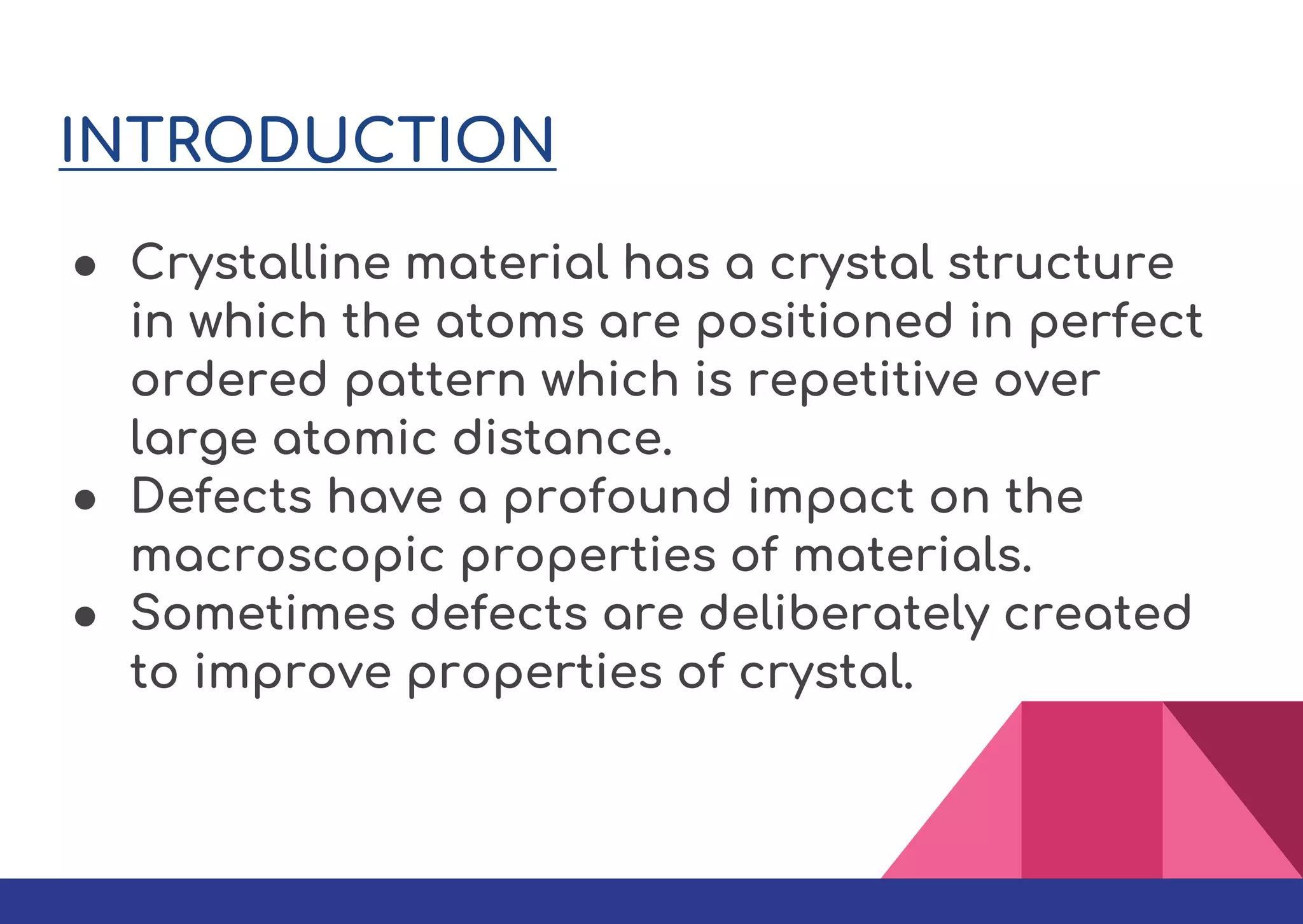
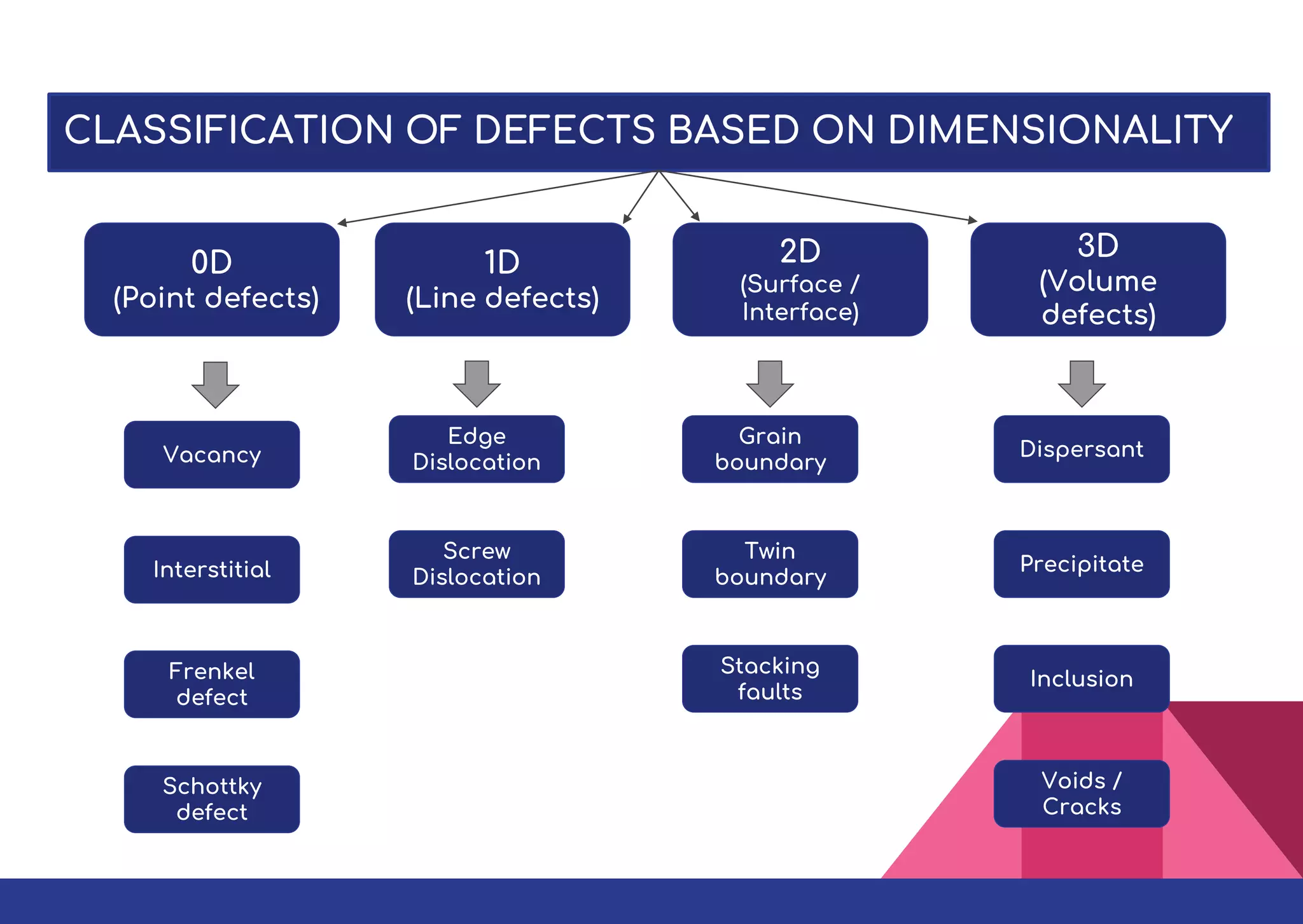
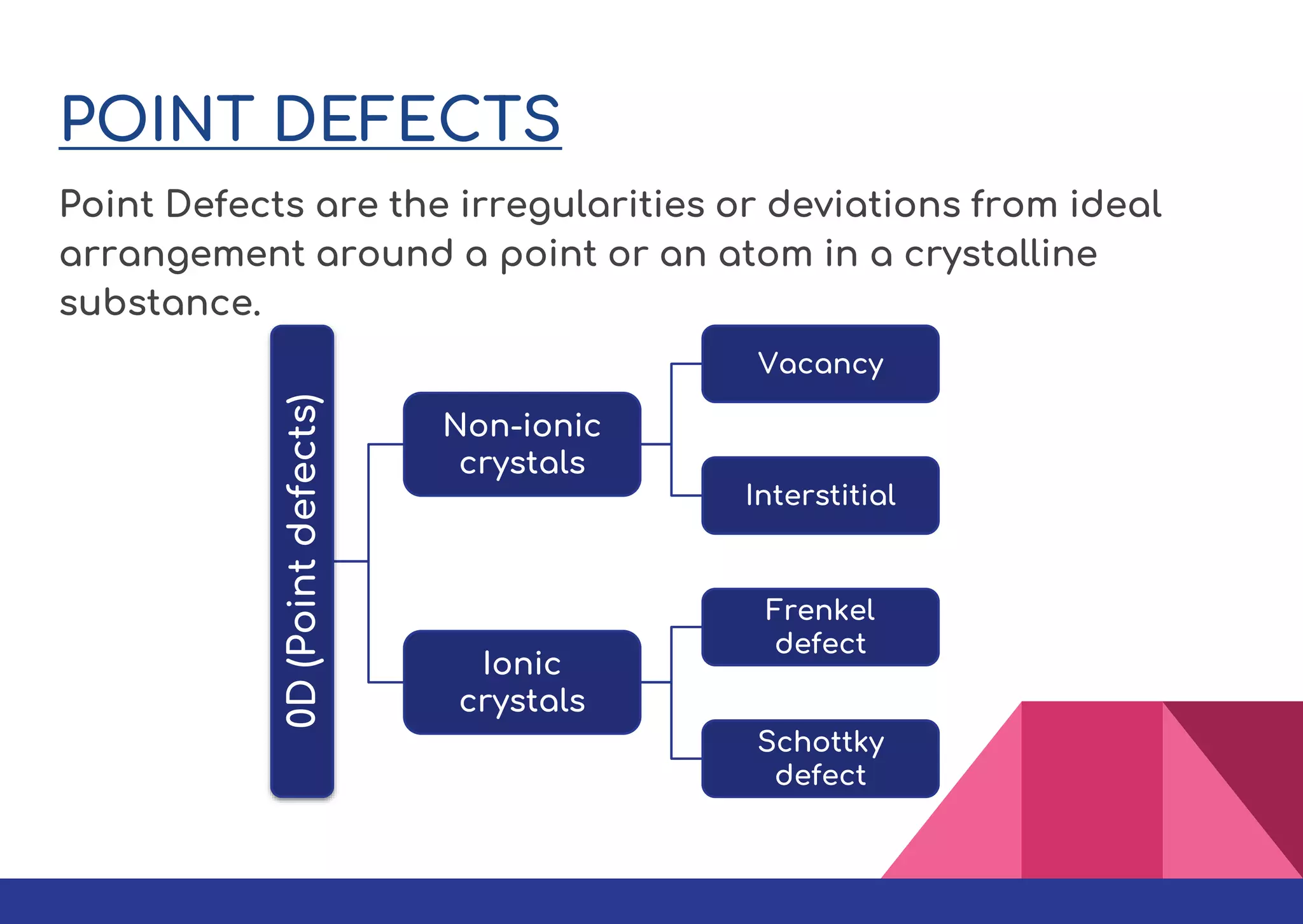
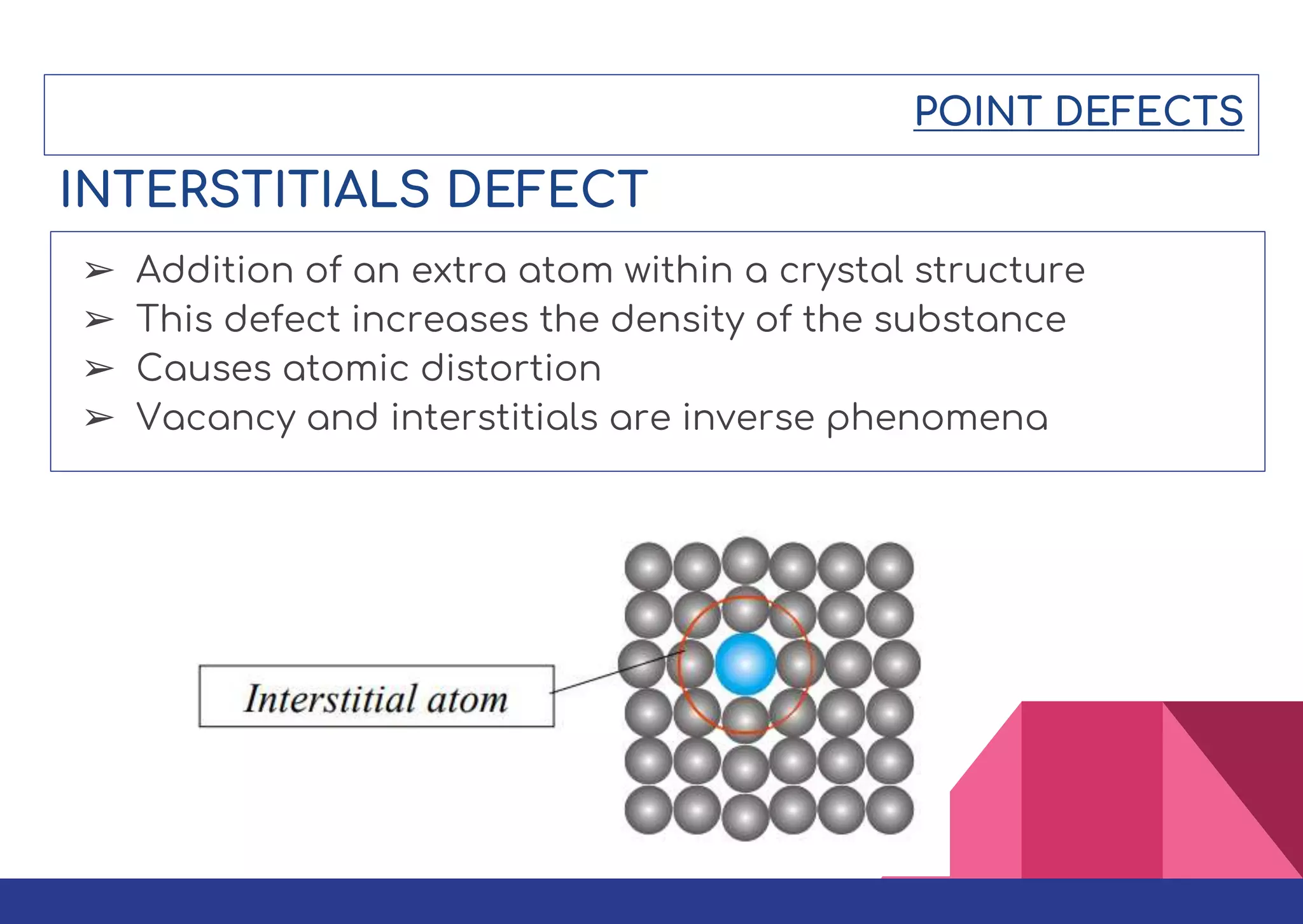
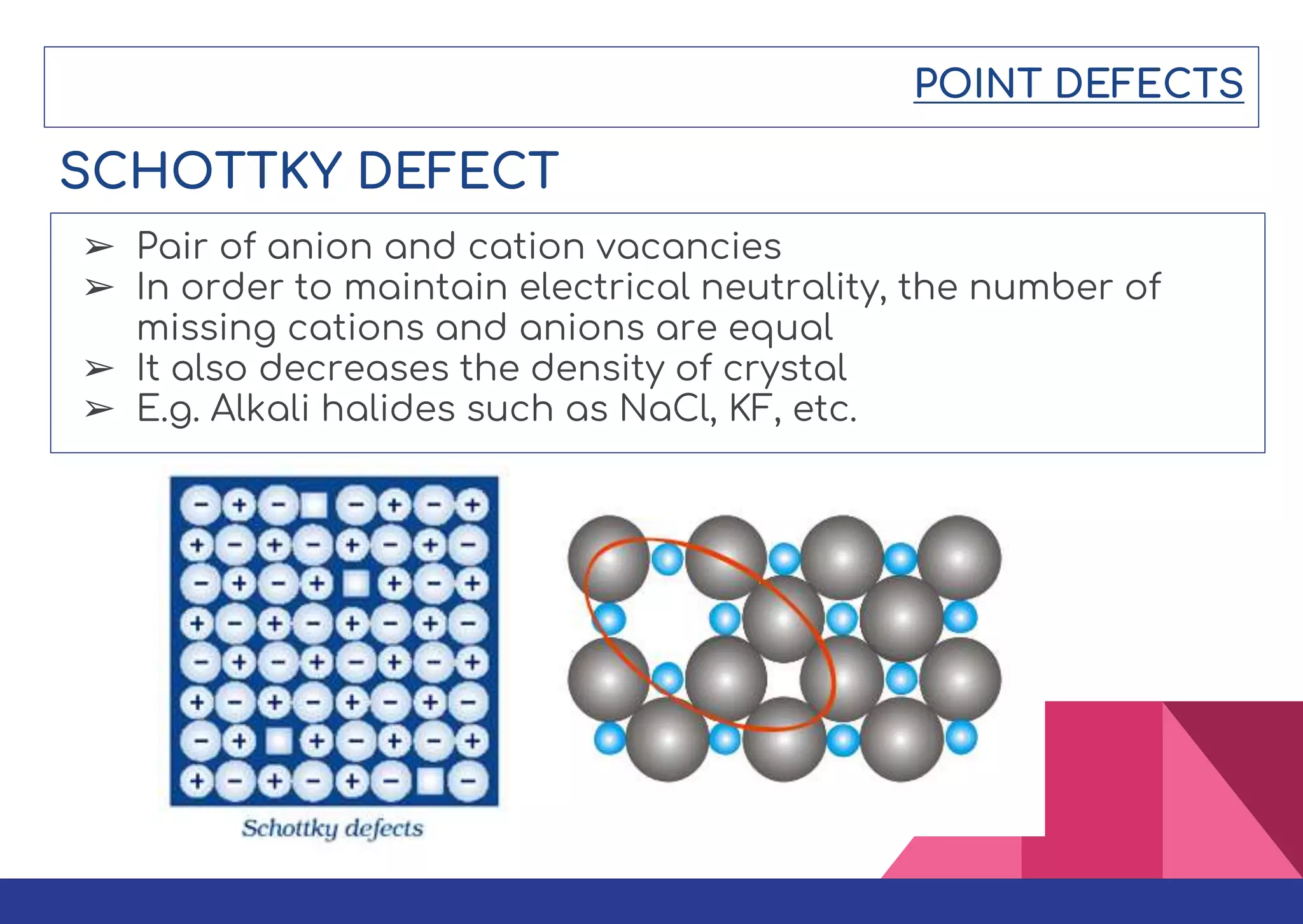
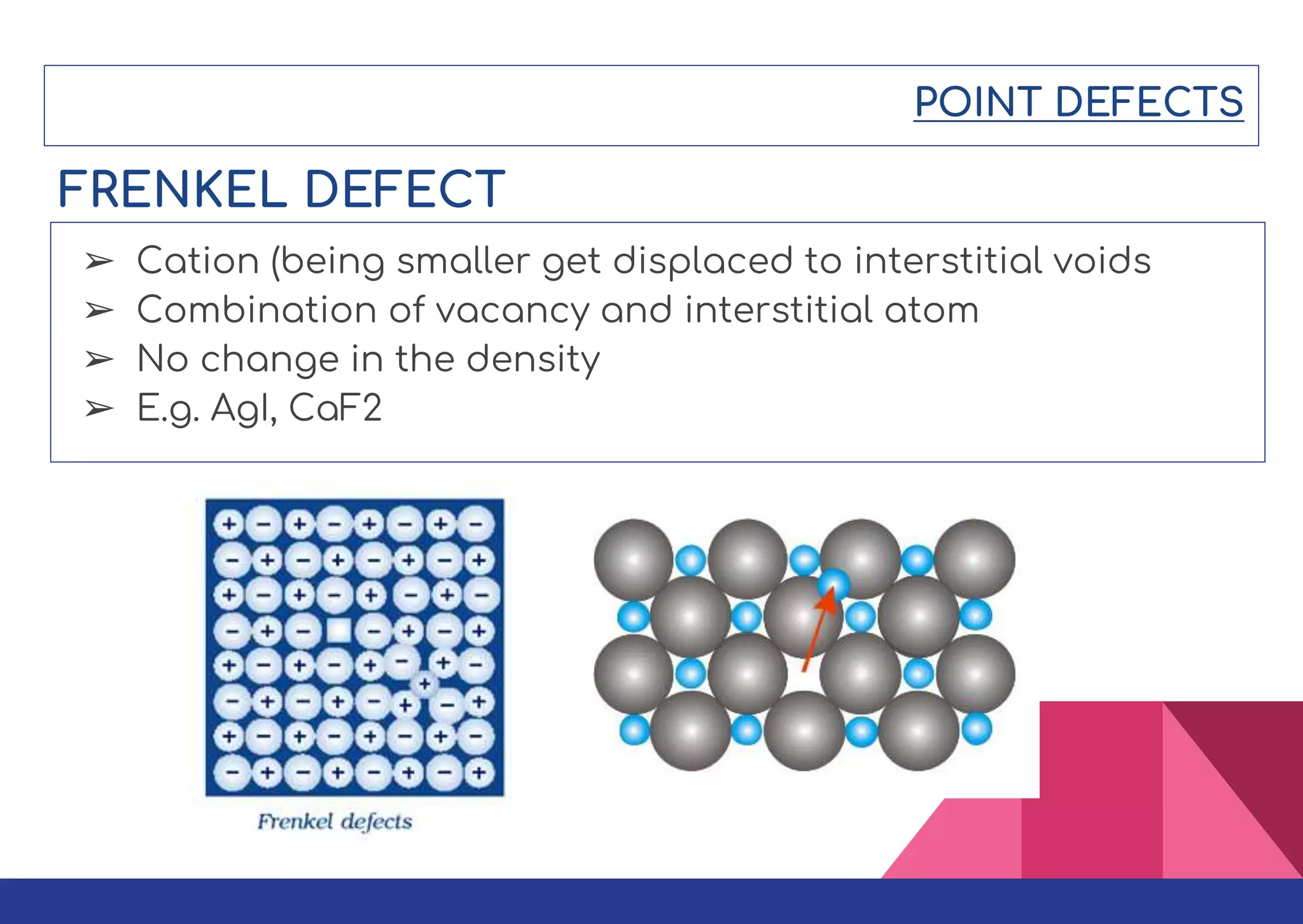
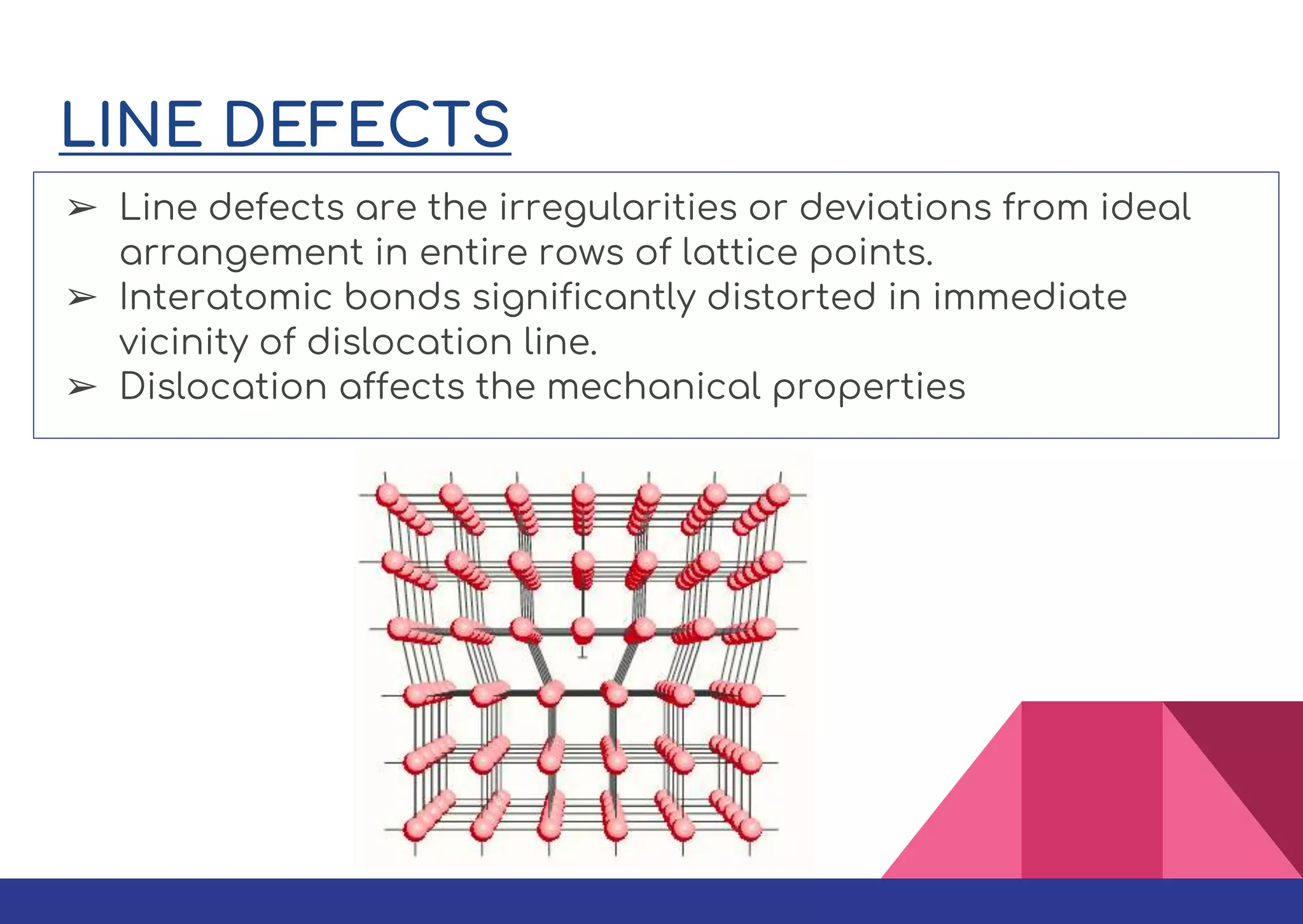
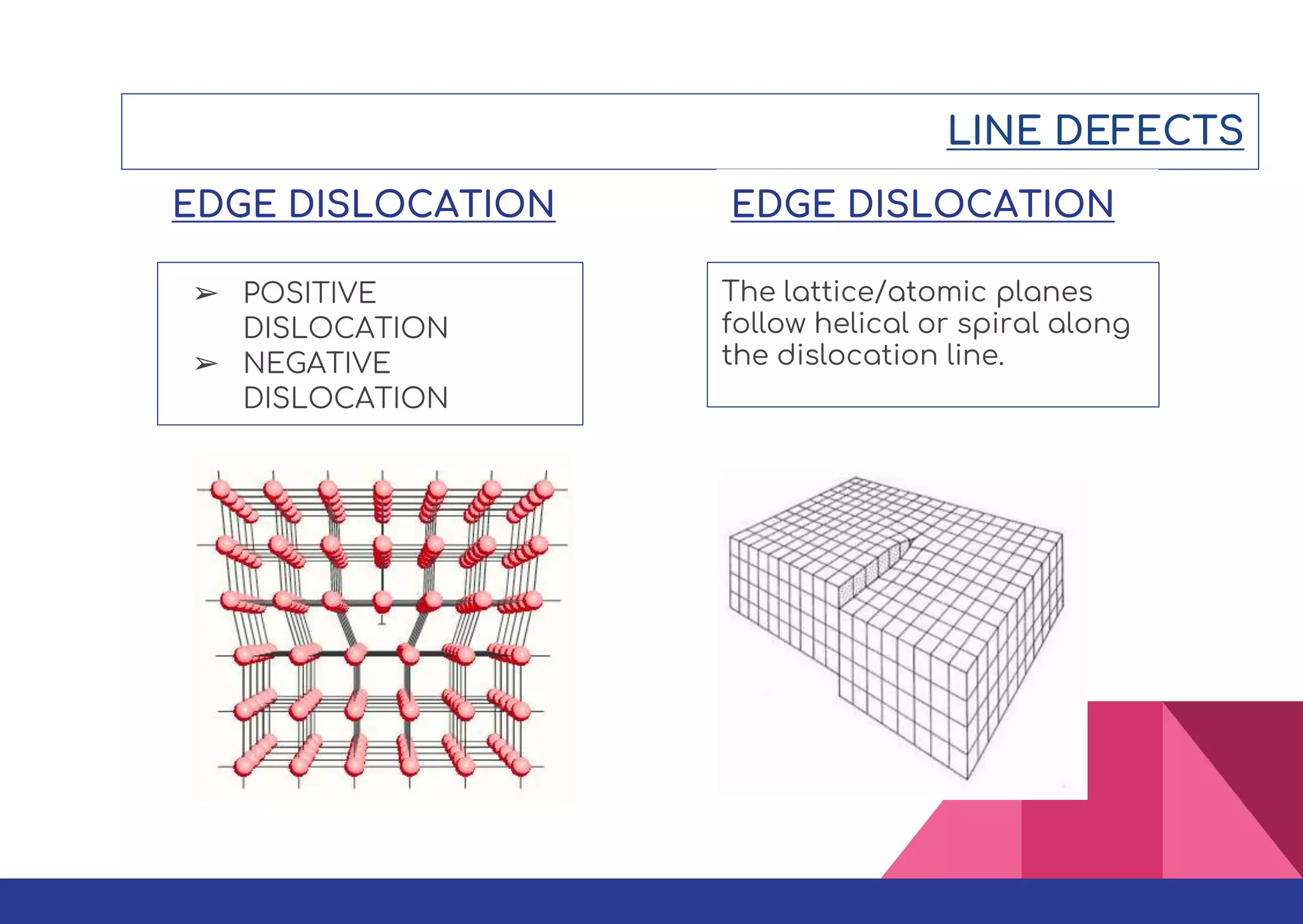
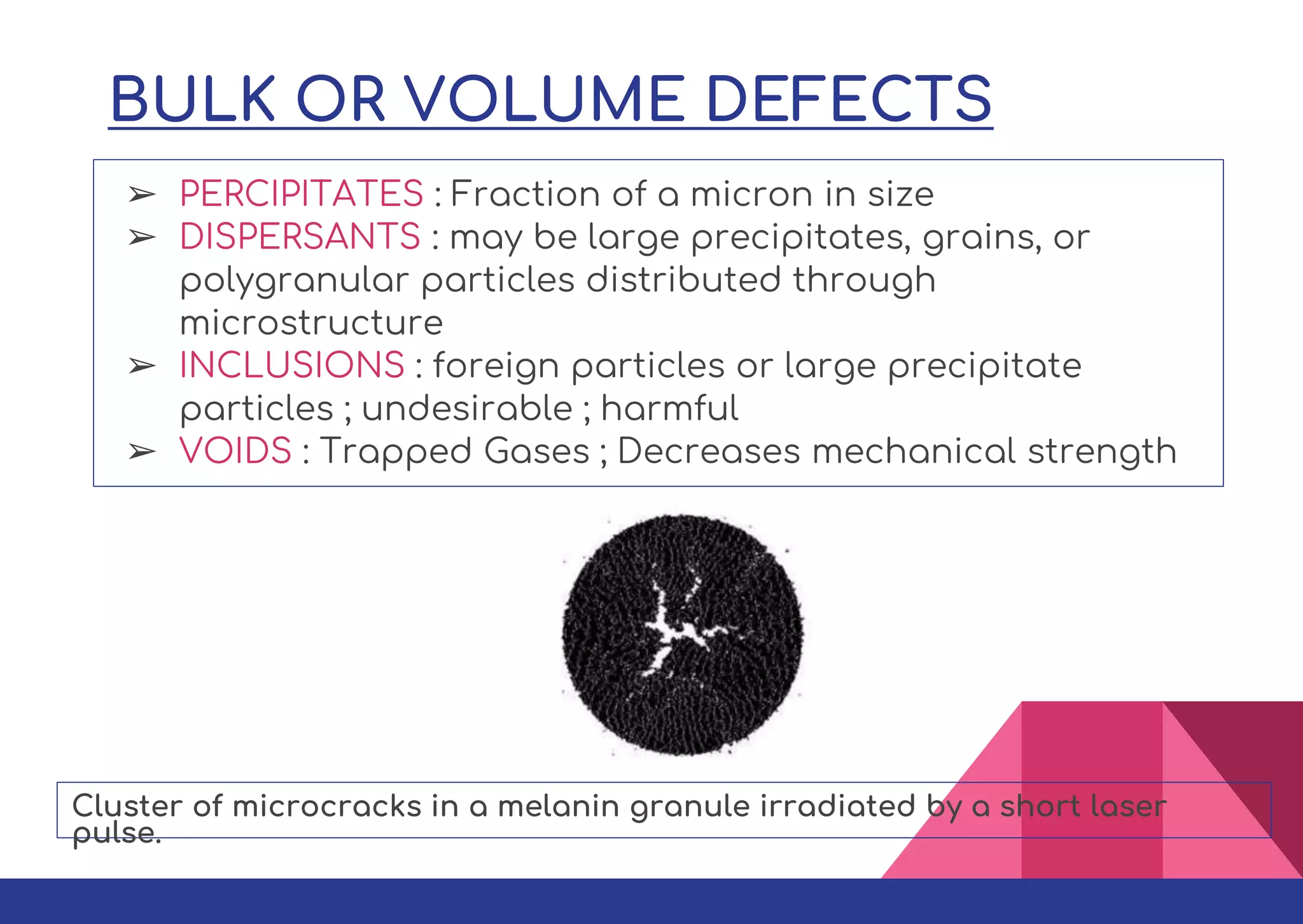
This document summarizes different types of defects found in crystals. It classifies defects based on dimensionality as point defects, line defects, surface defects, and bulk defects. Point defects include vacancies, interstitials, Schottky defects, and Frenkel defects. Line defects are dislocations that can be edge or screw dislocations. Surface defects occur at grain boundaries due to changes in crystal structure. Bulk defects include precipitates, dispersants, inclusions, and voids. Defects can impact material properties and are sometimes deliberately introduced to improve properties.
![SETH MOTILAL [P.G.] COLLEGE,
JHUNJHUNU
SEMINAR
M.Sc. FINAL CHEMISTRY
Submitted to:
Prof. Babita (H.O.D.)
Submitted by :
Ashish Kumar Sonkaria
Roll No. - 22335106](https://image.slidesharecdn.com/defectsincrystals-230407124908-03d3d2d6/75/DEFECTS-IN-CRYSTALS-pptx-1-2048.jpg)