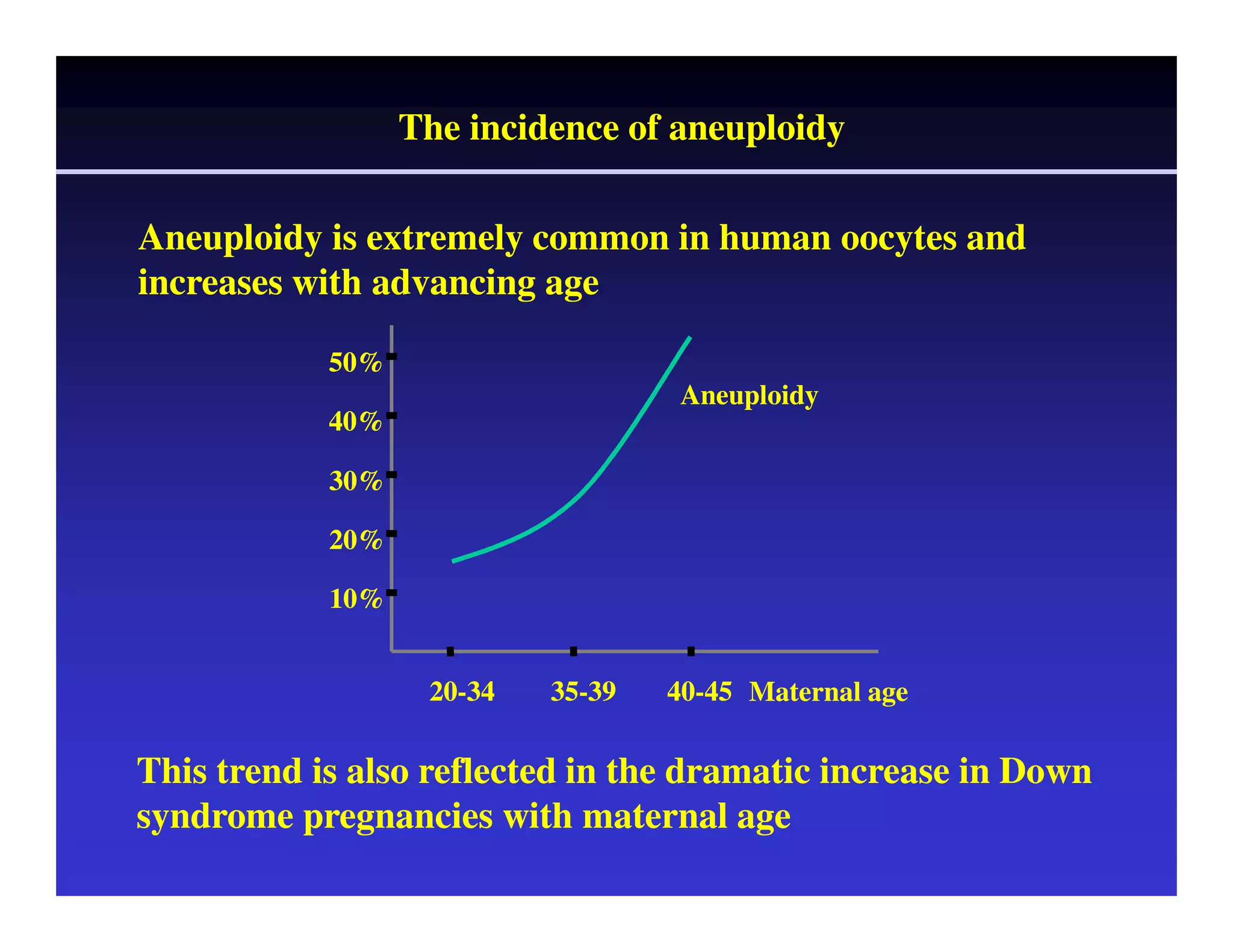
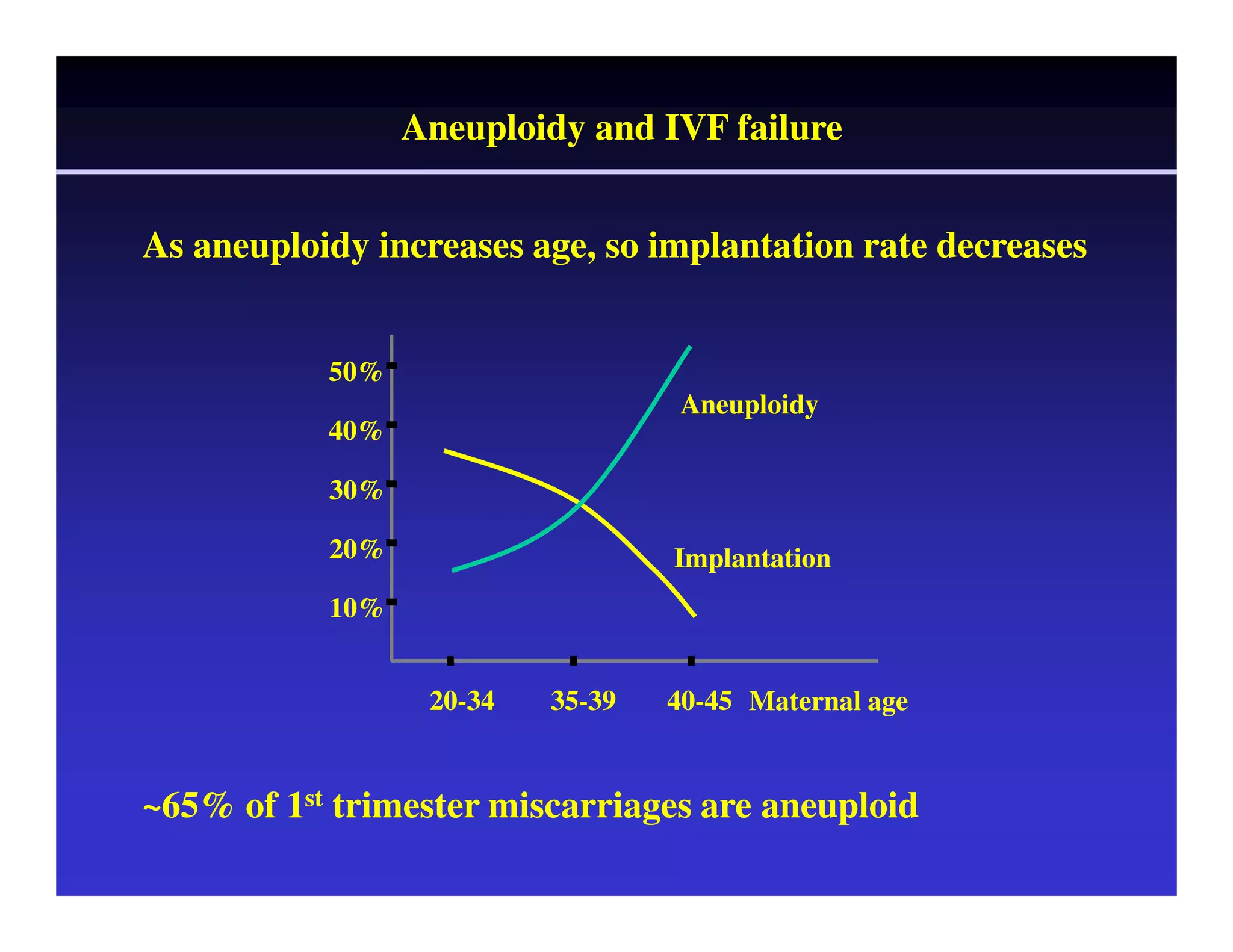
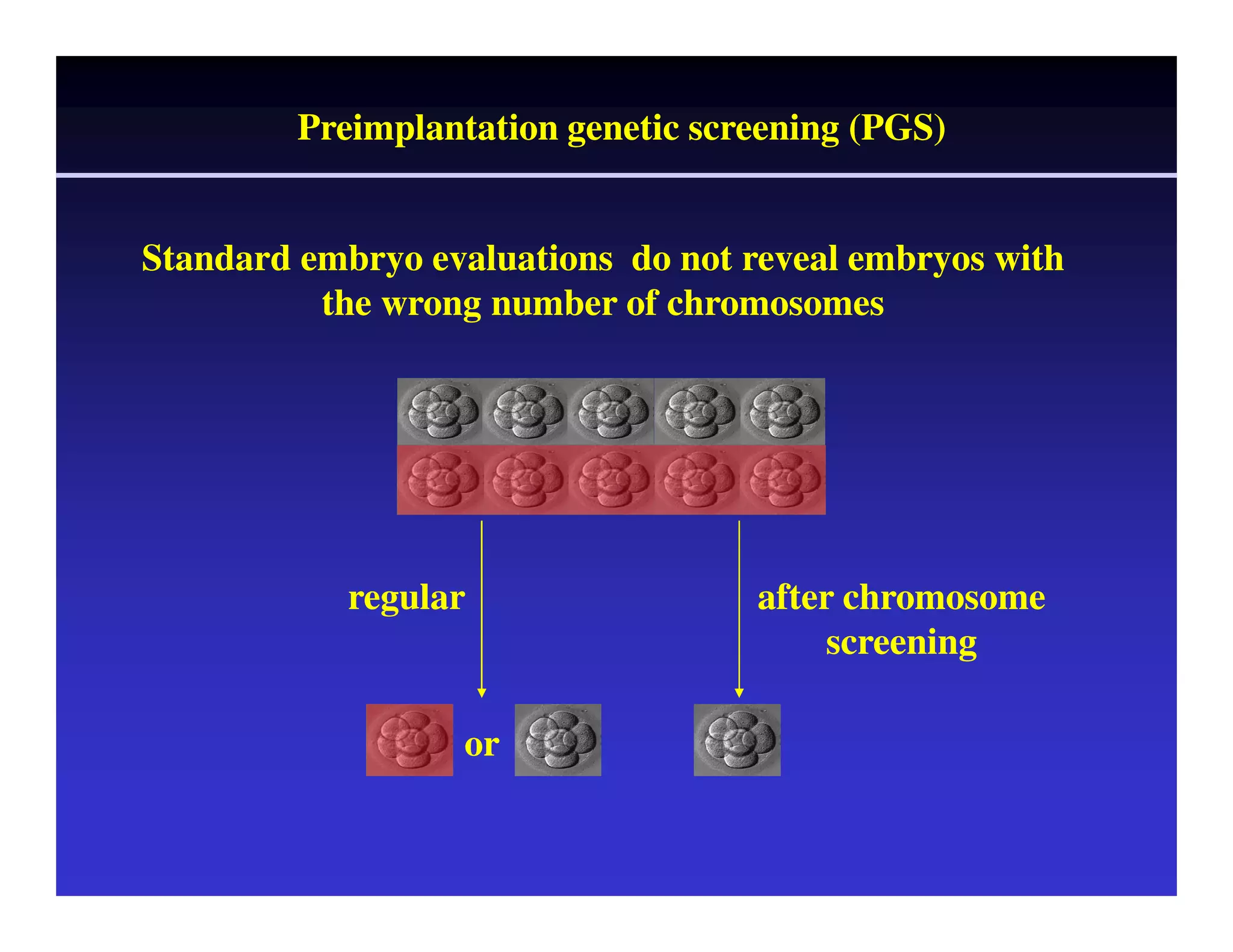
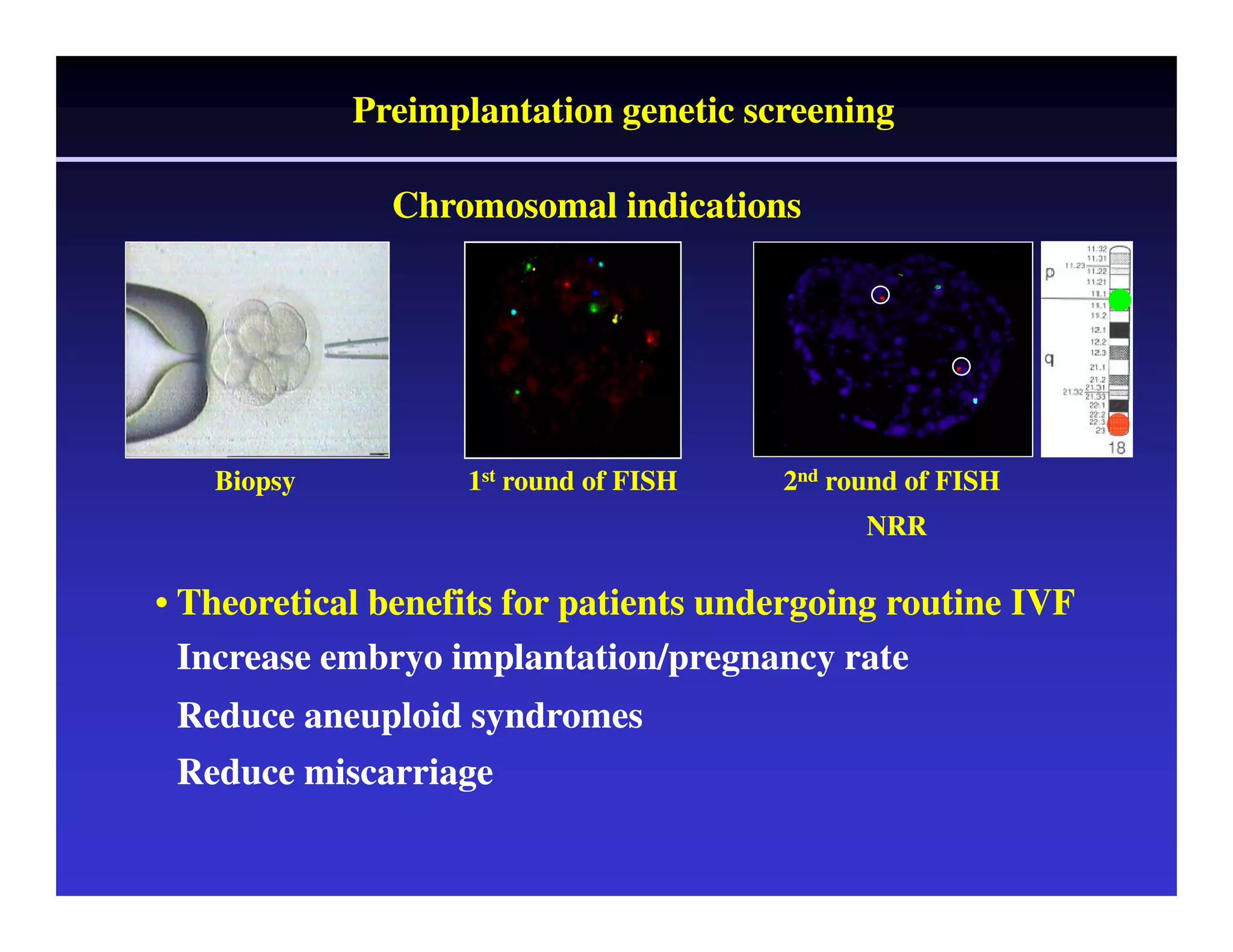
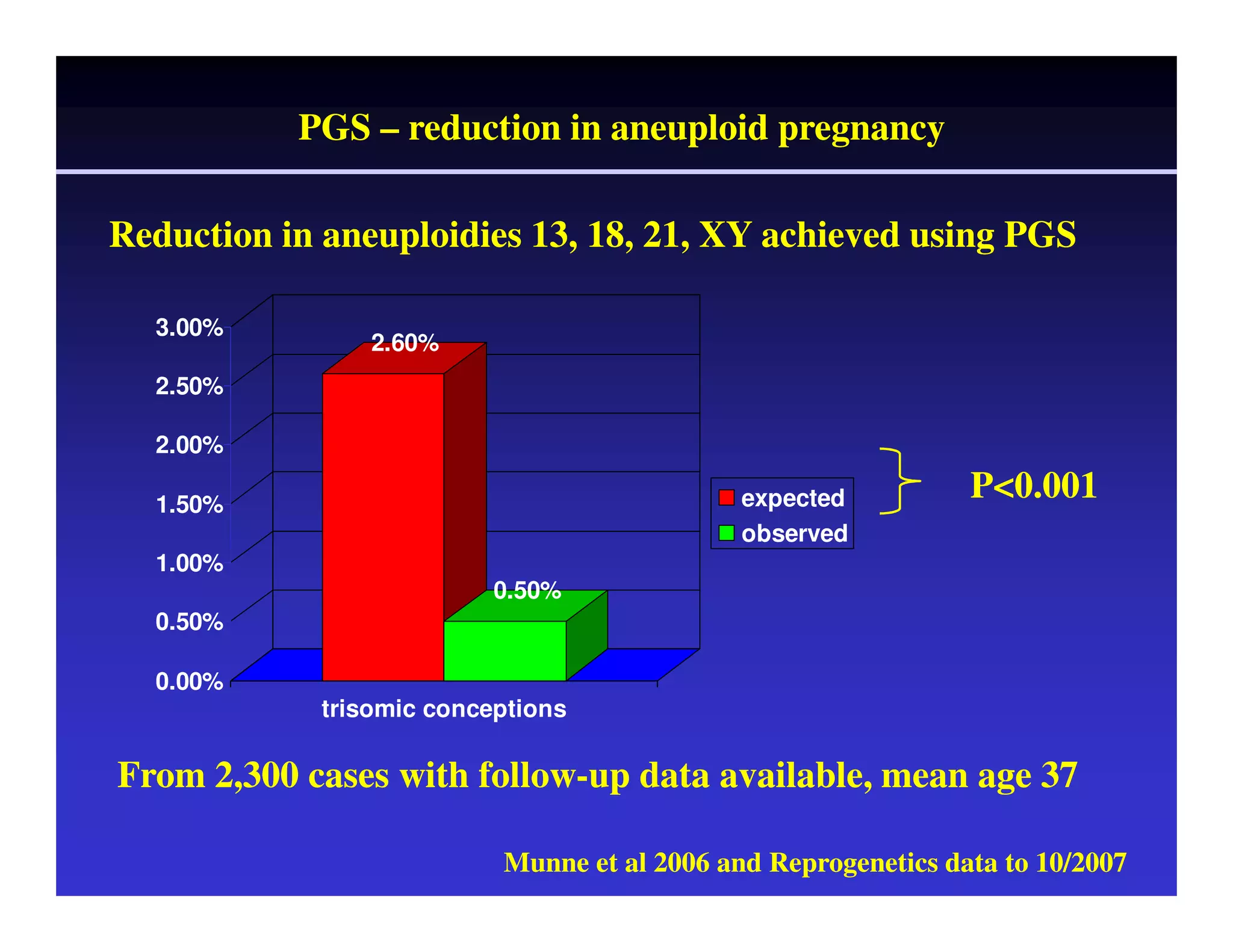
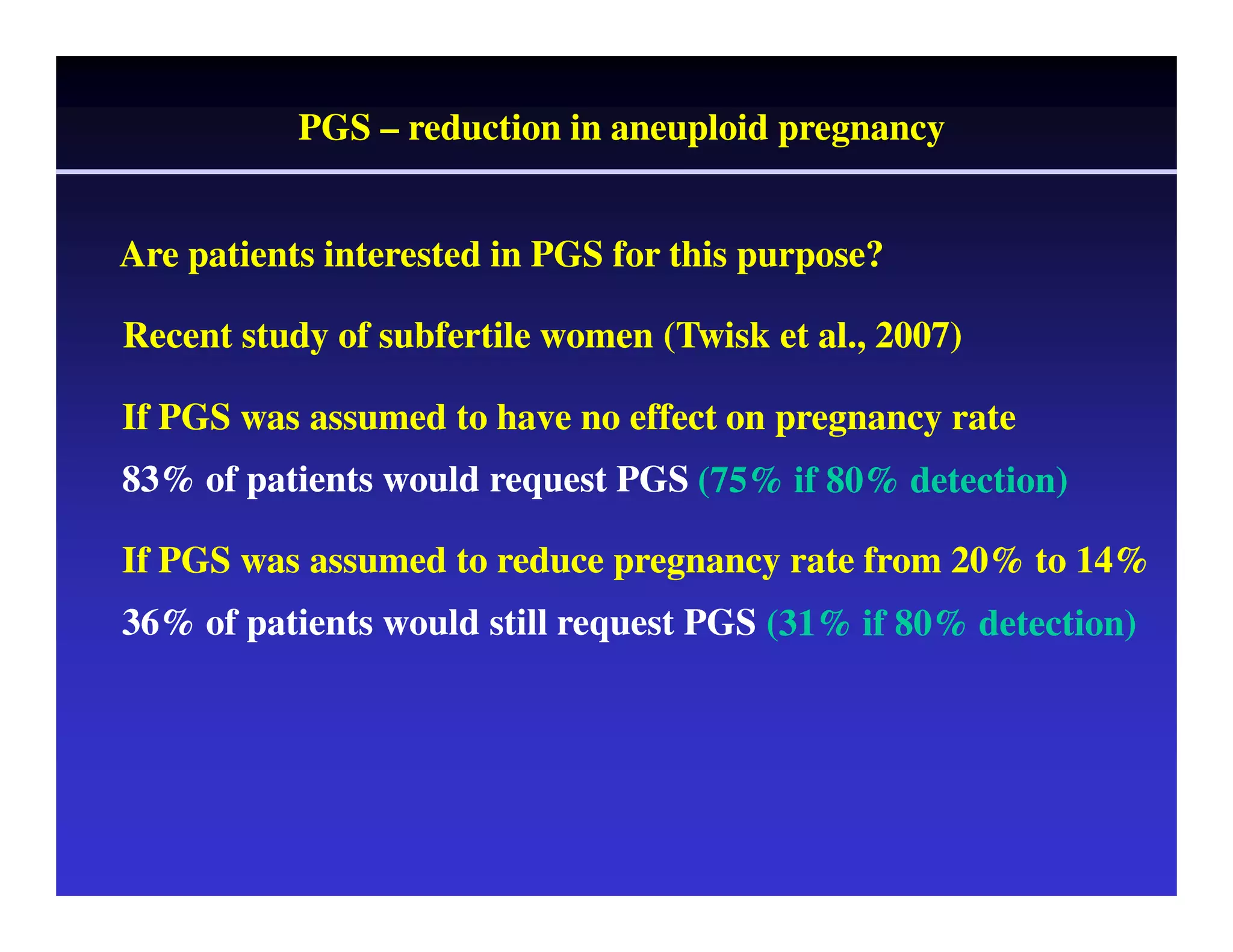
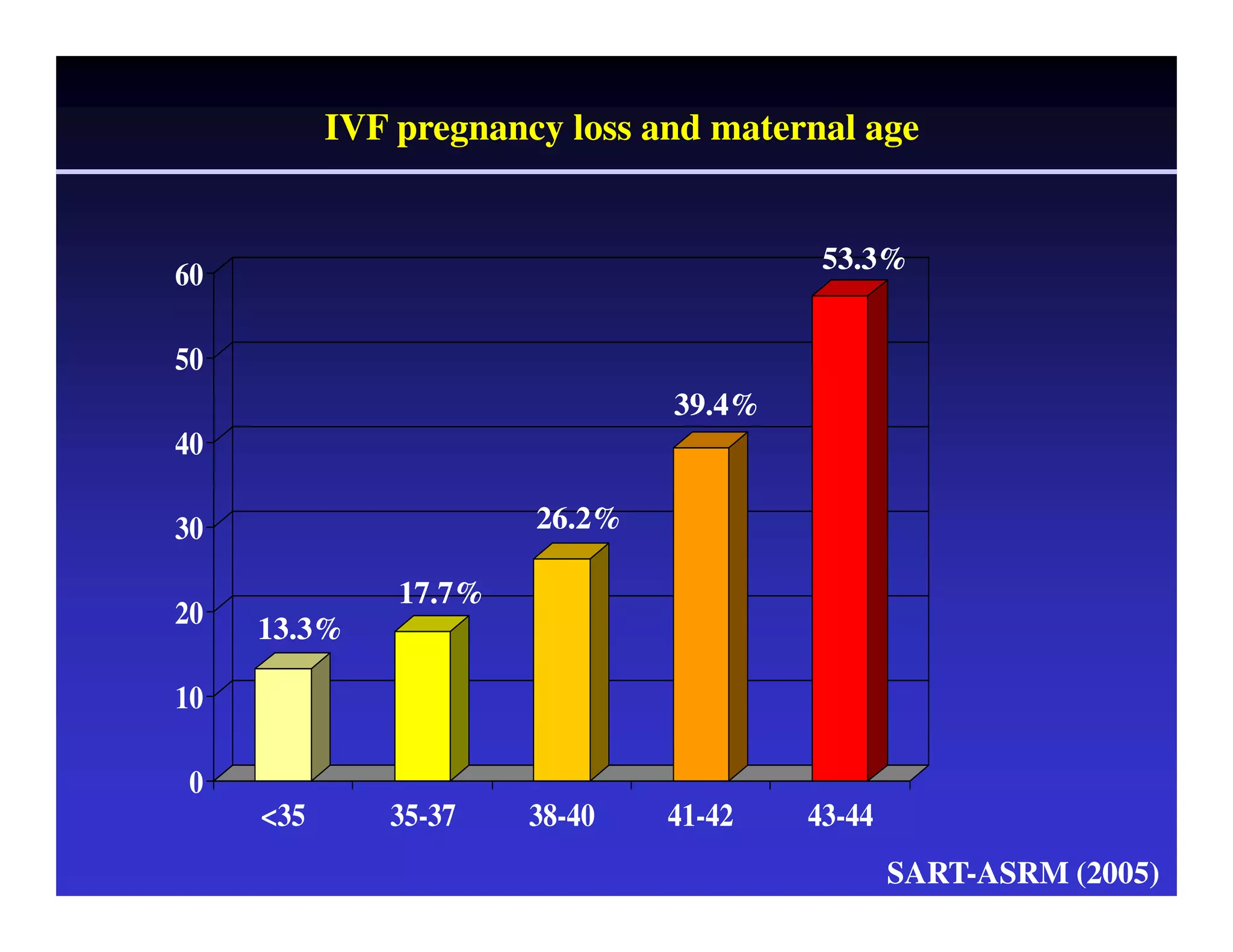
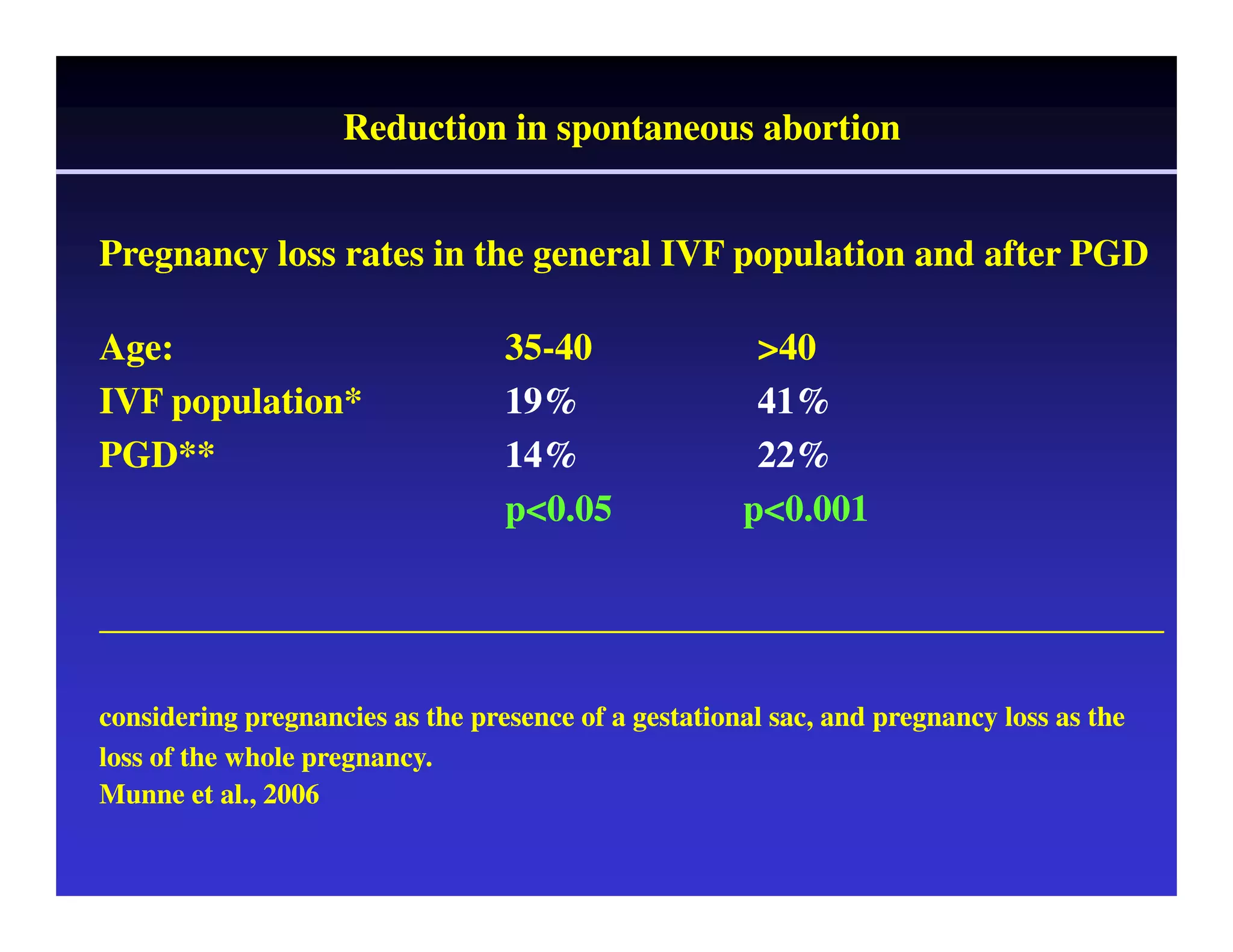
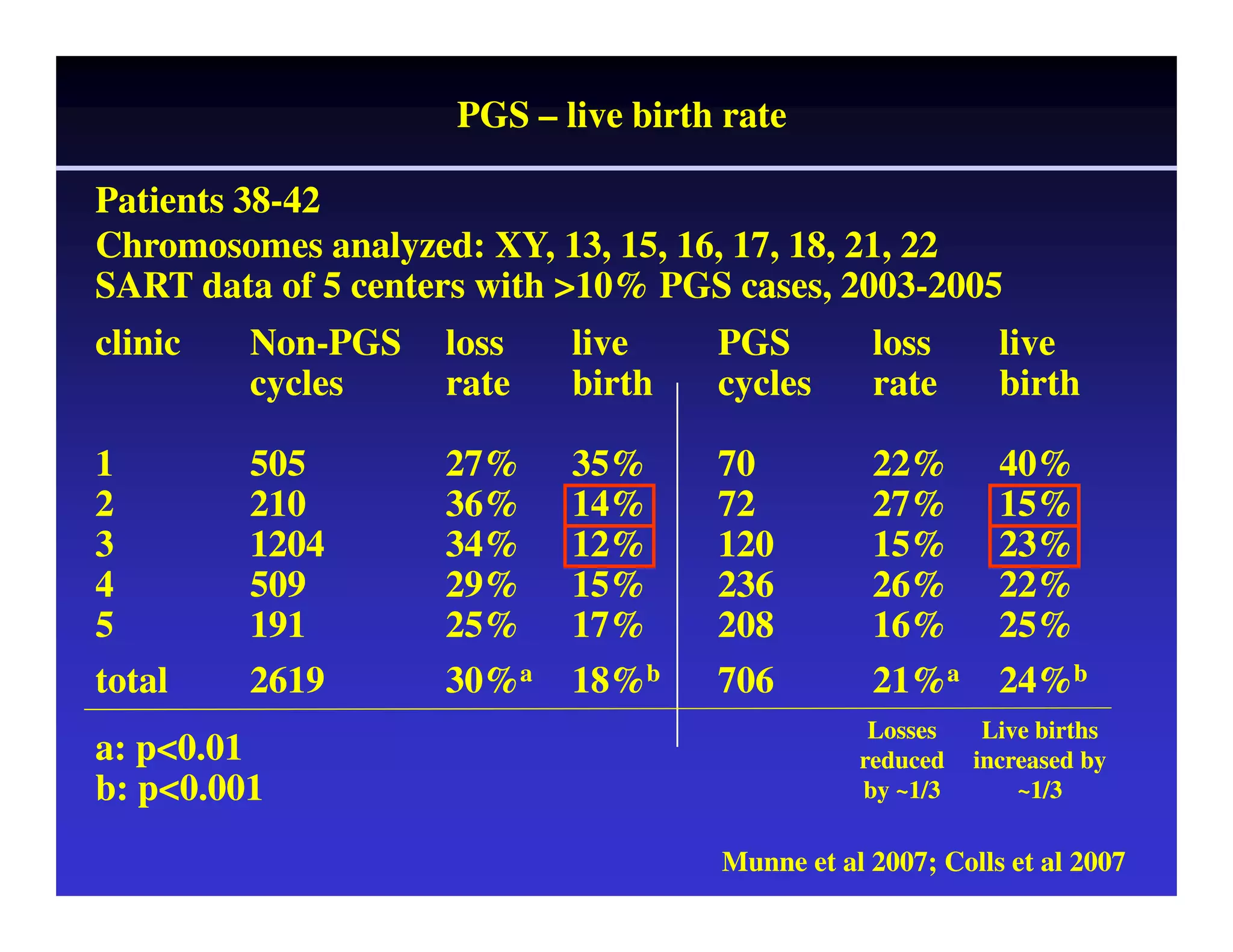
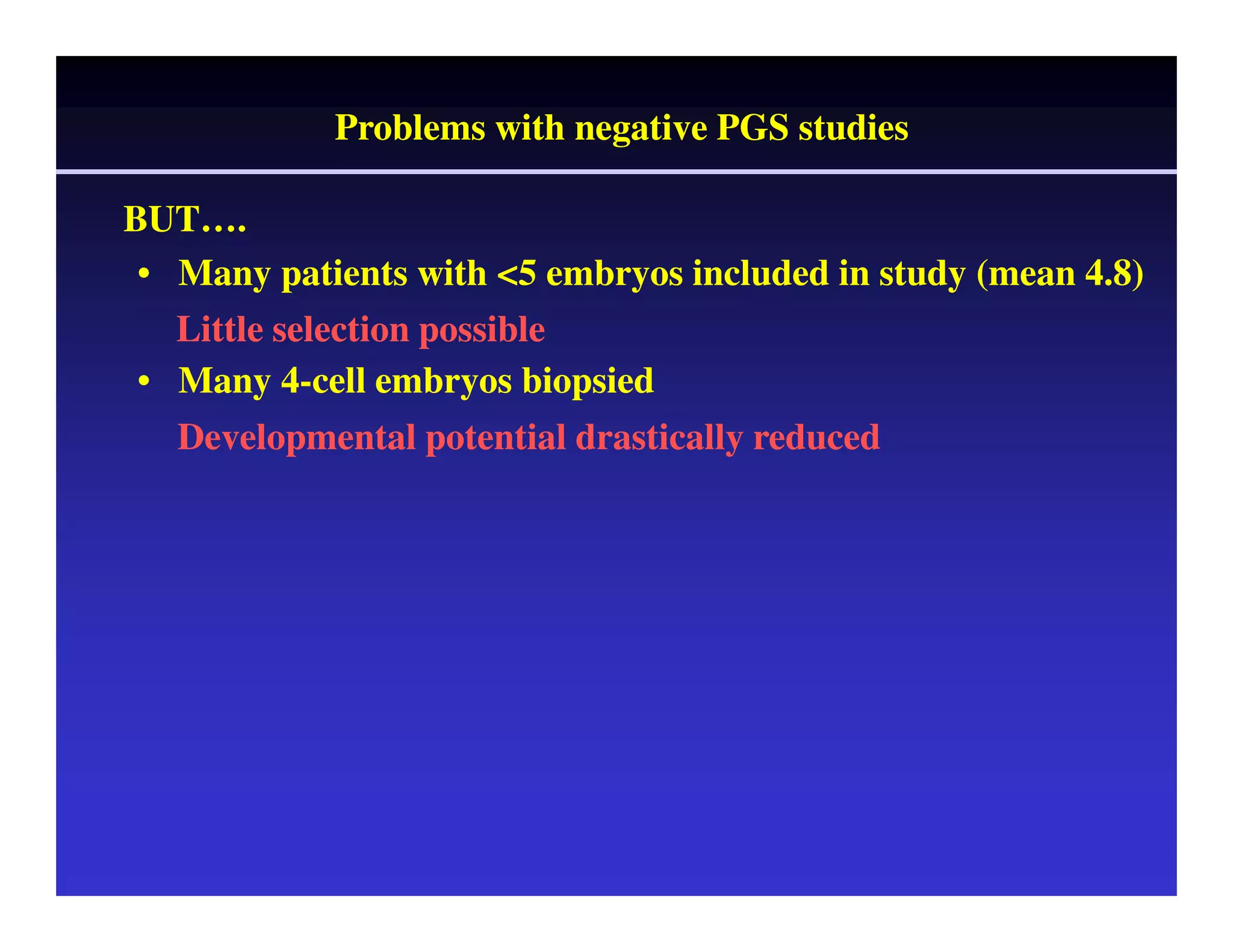
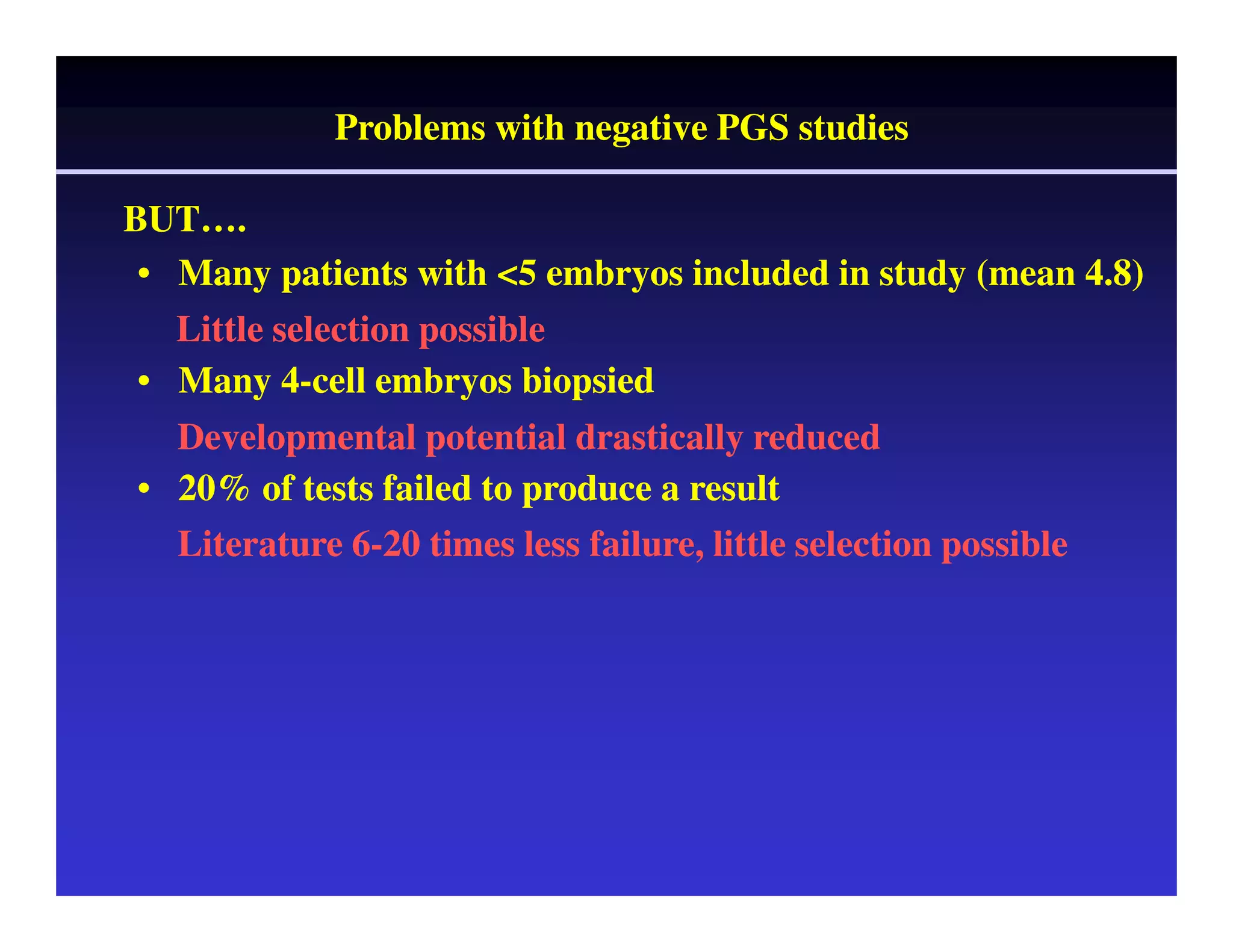
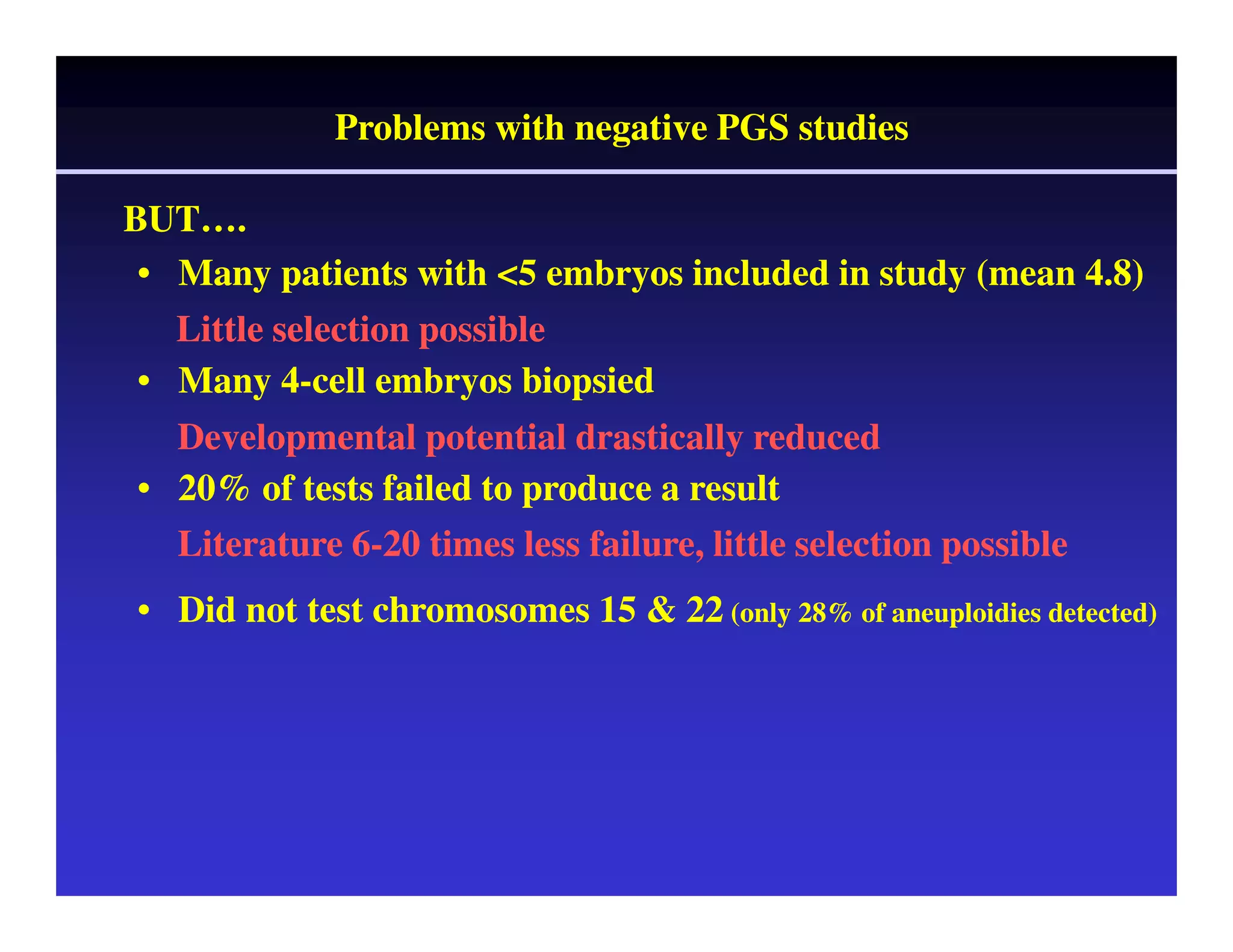
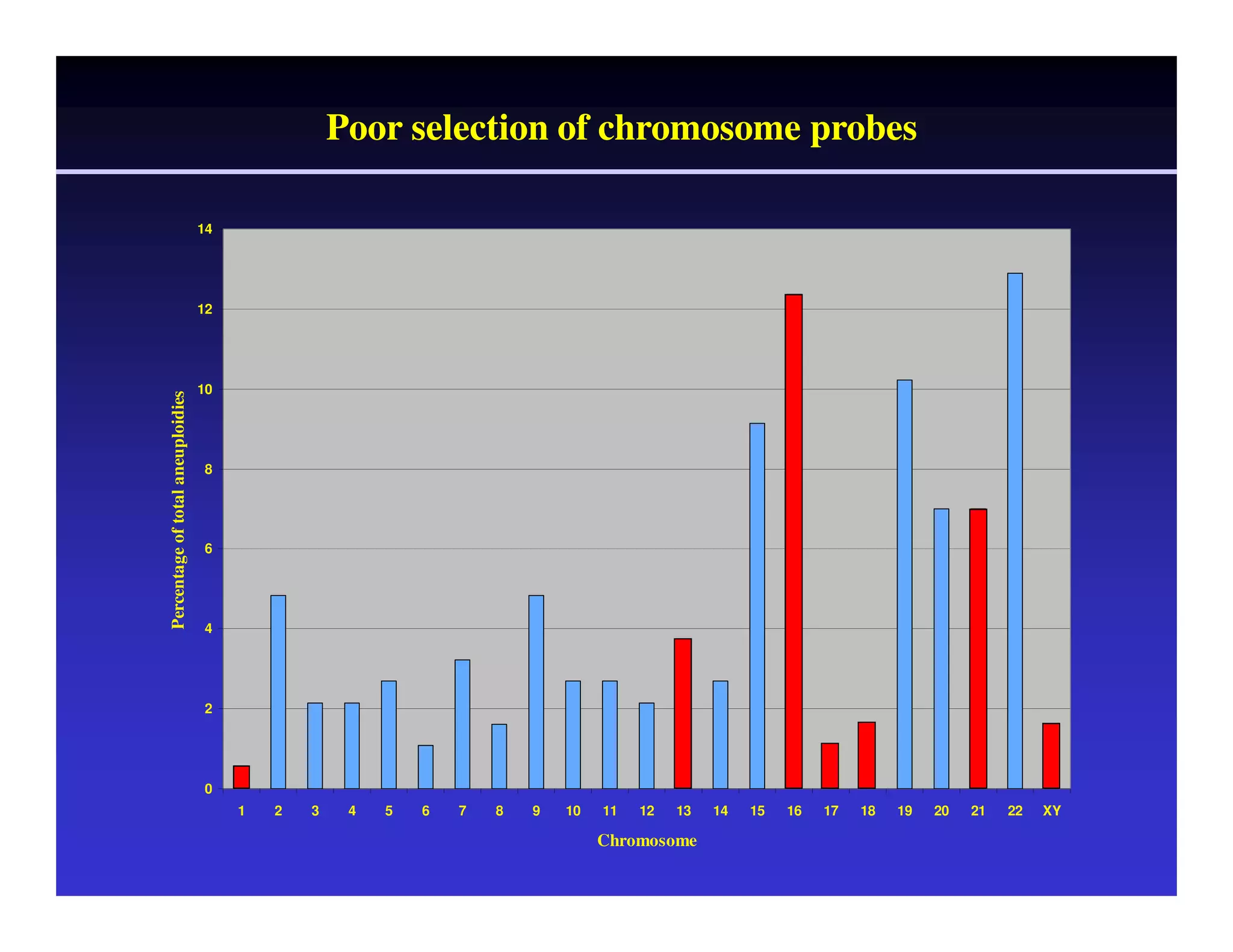
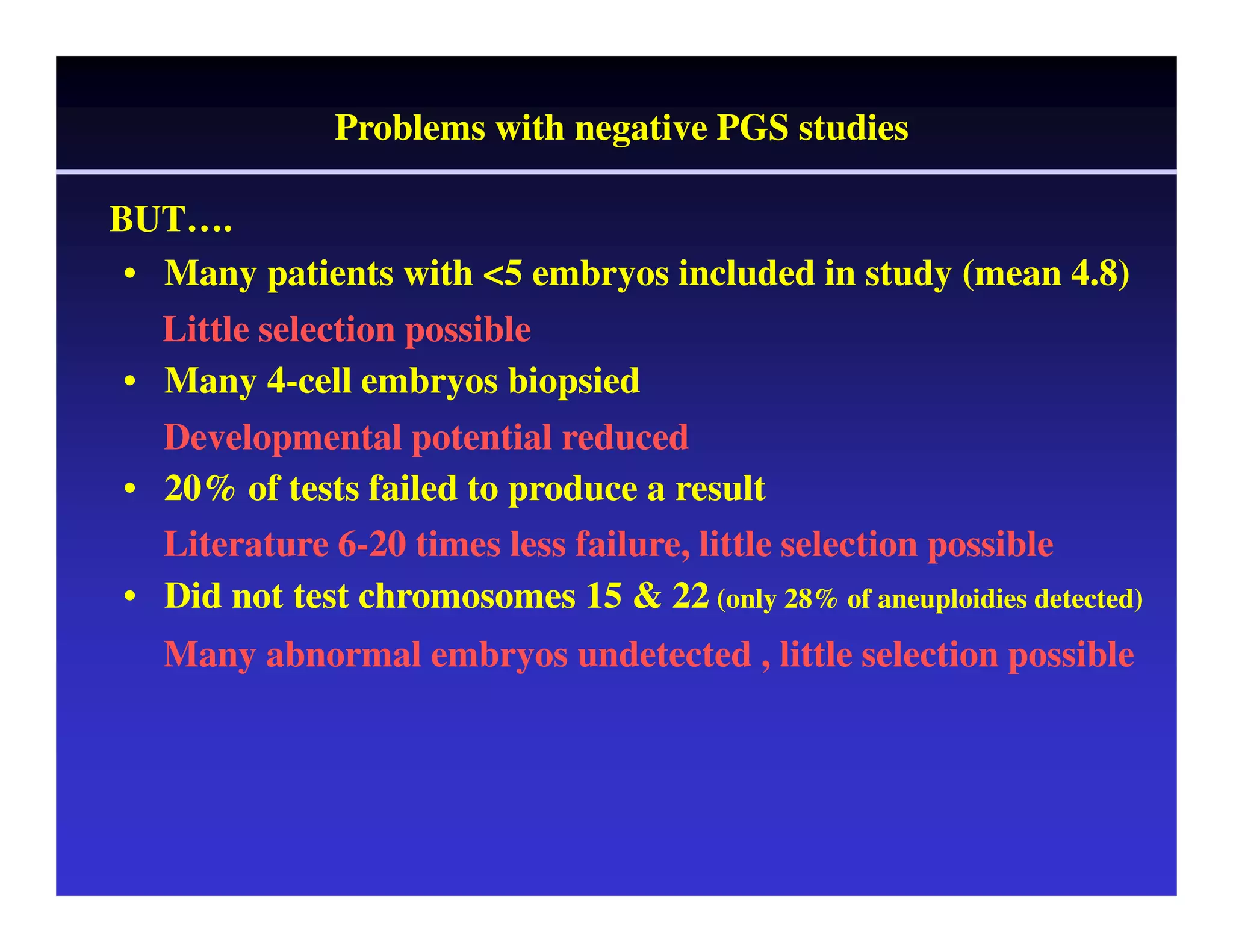
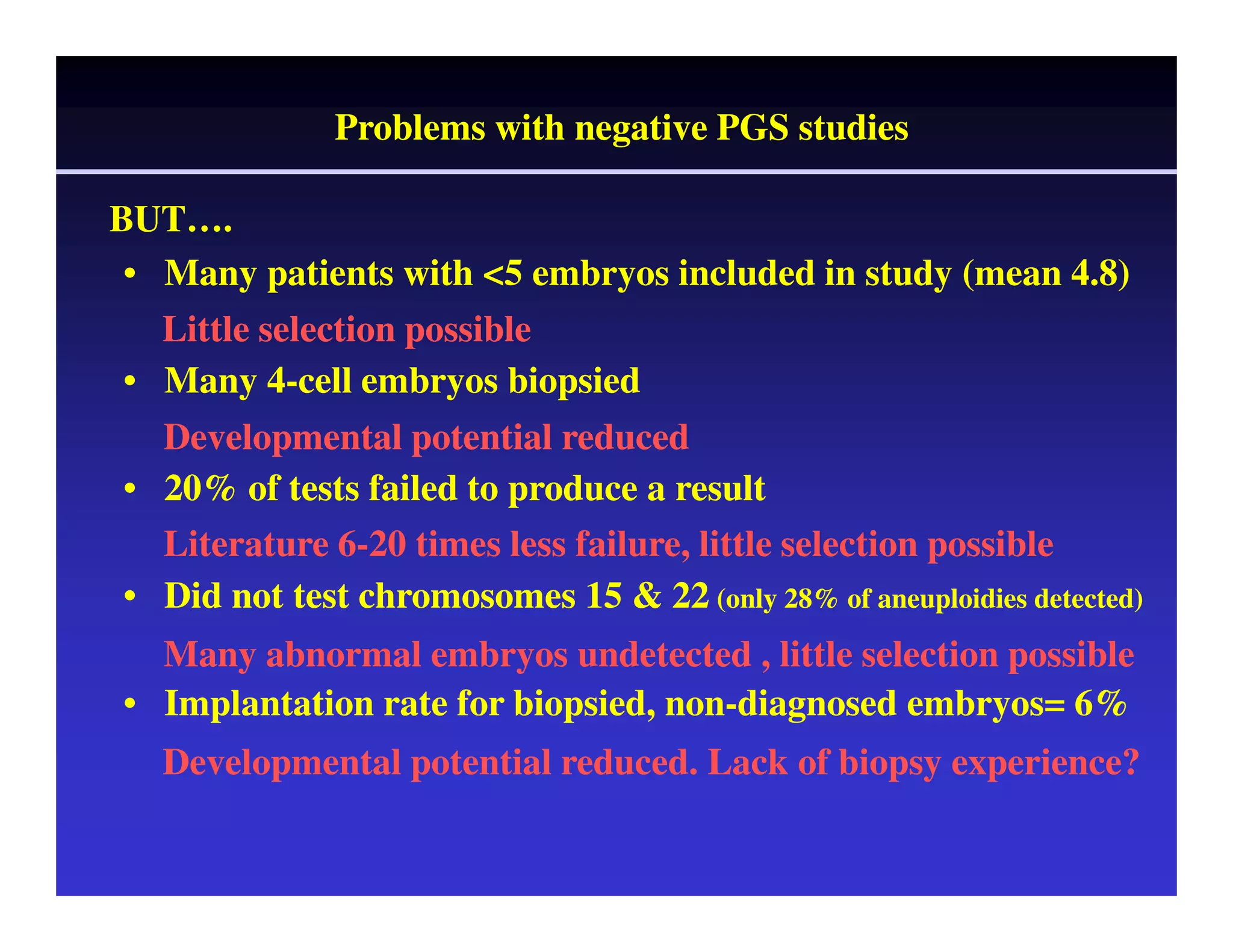
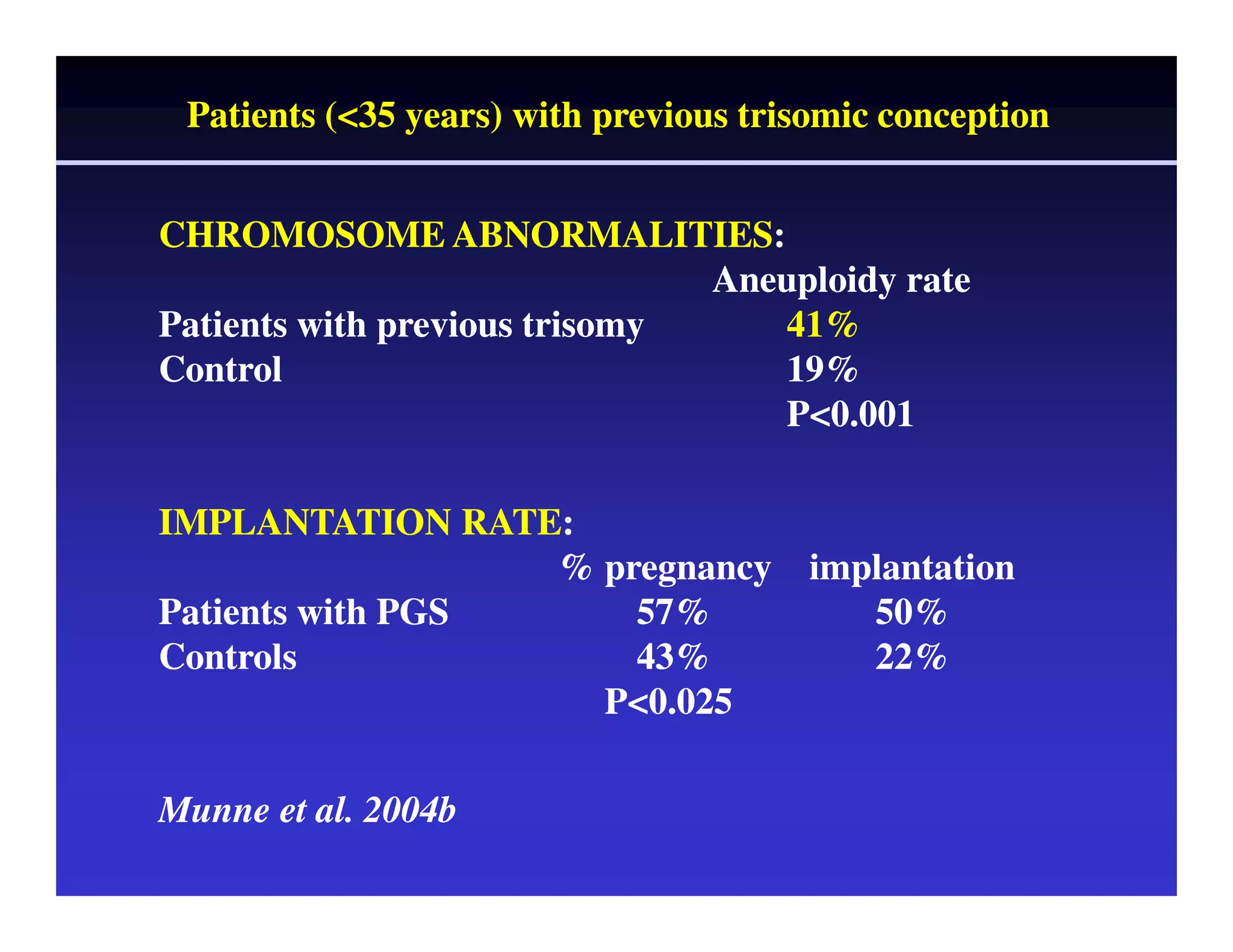
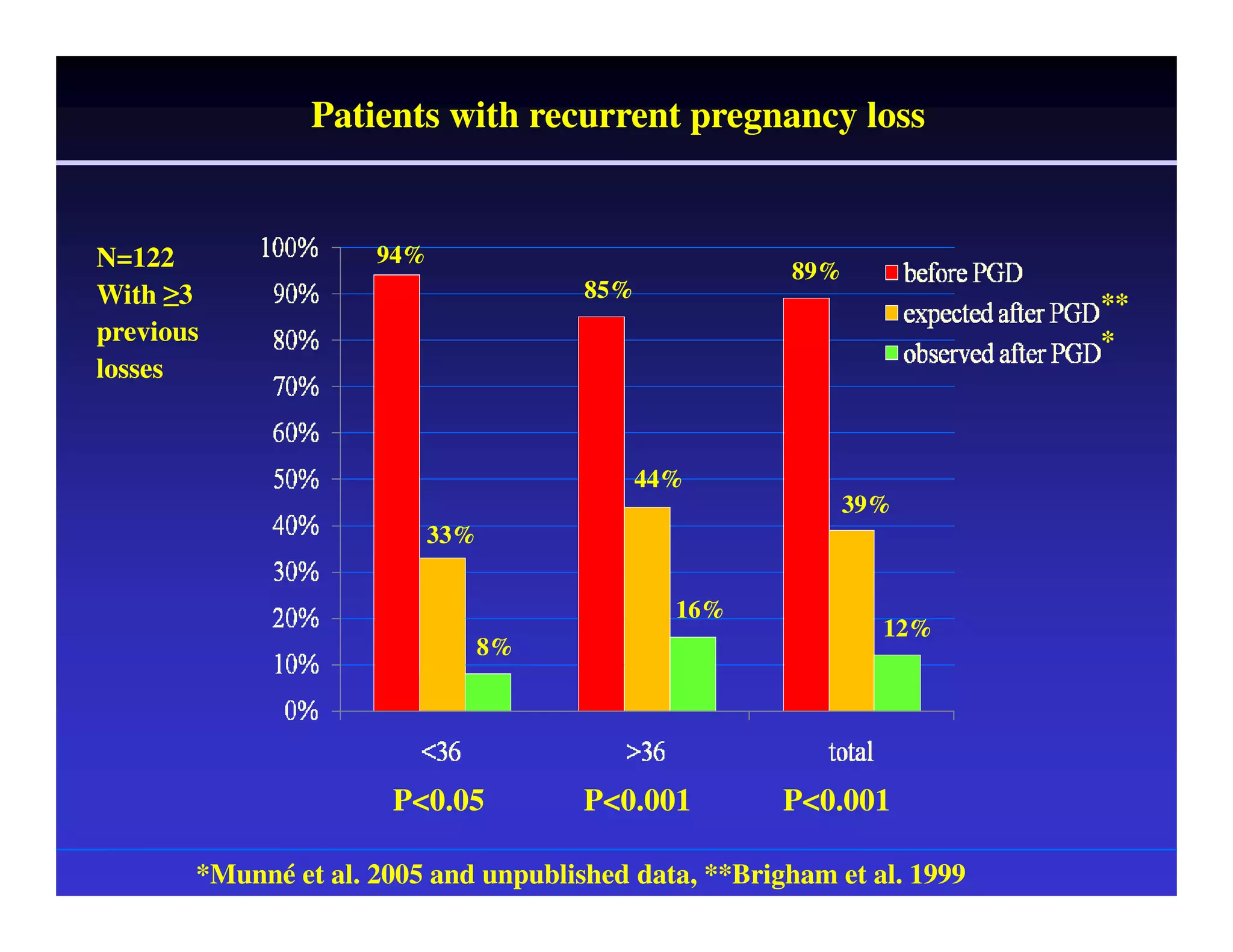
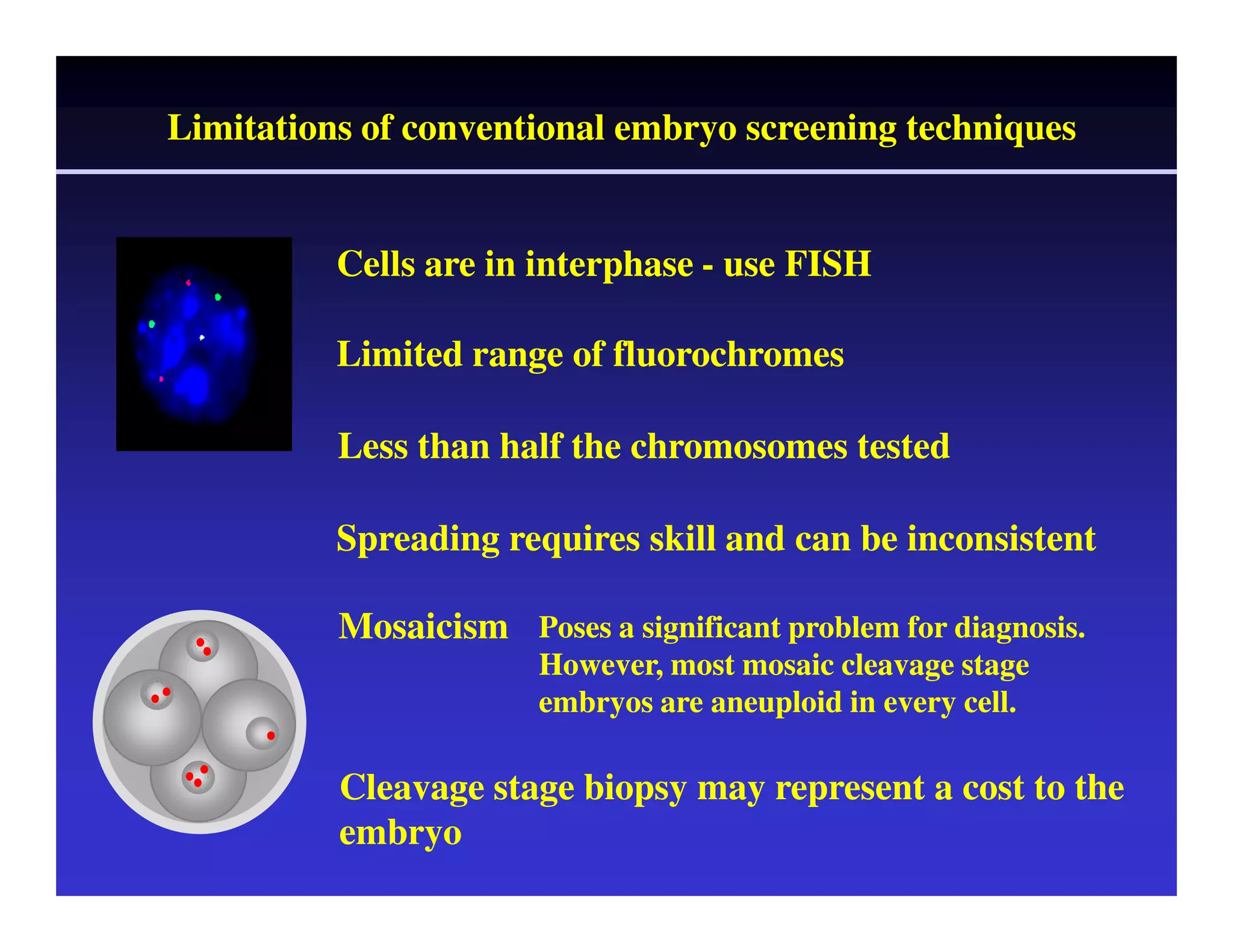
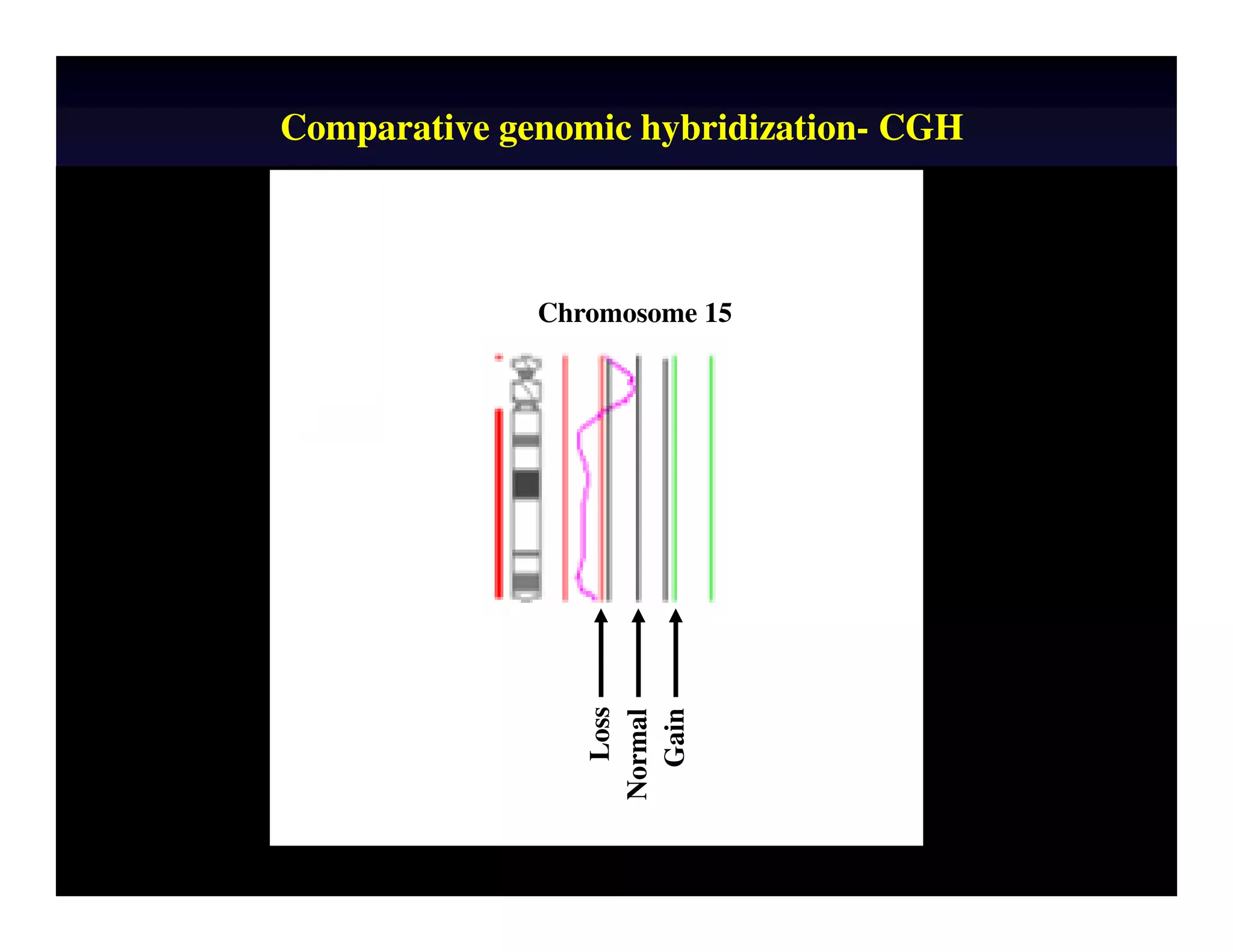
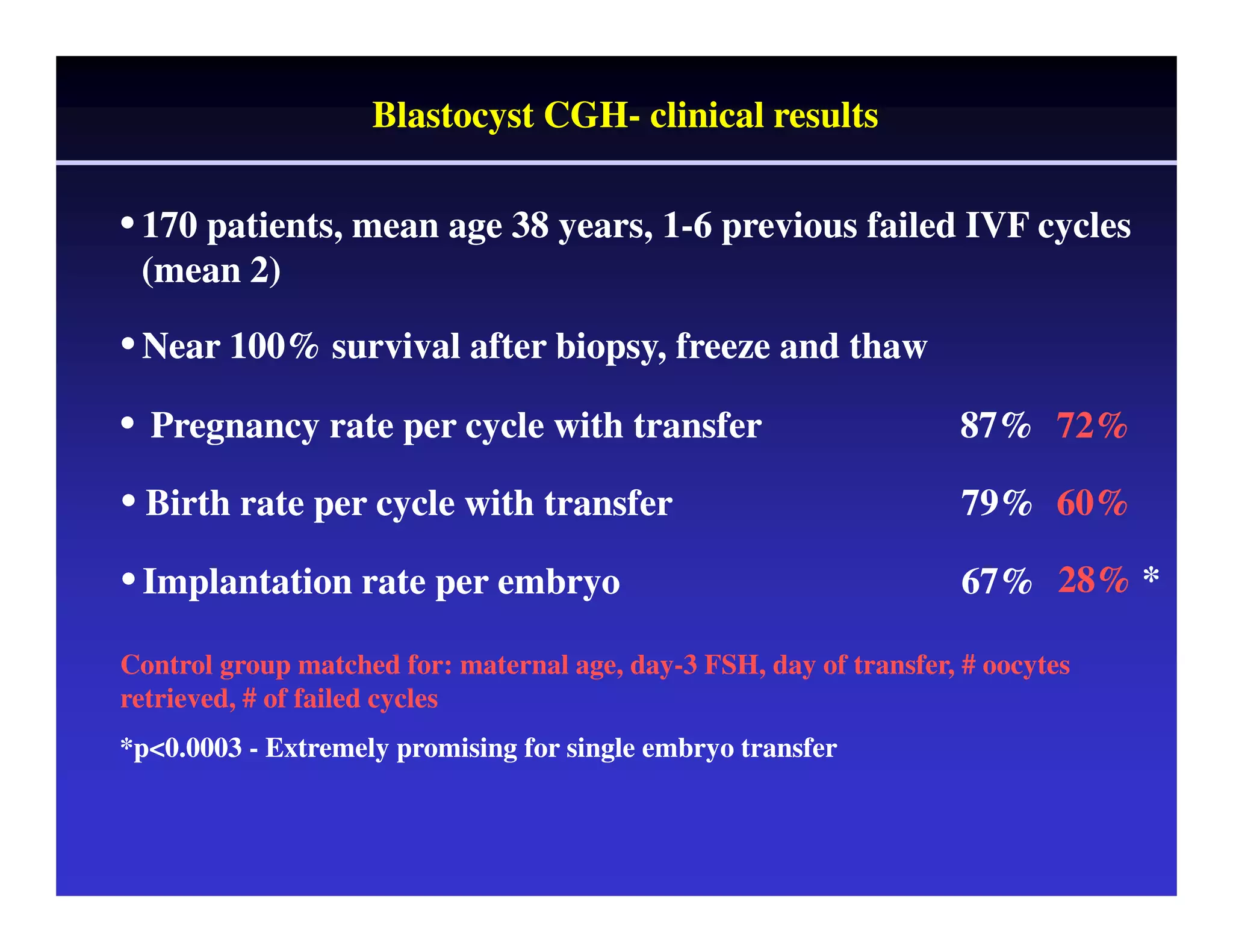
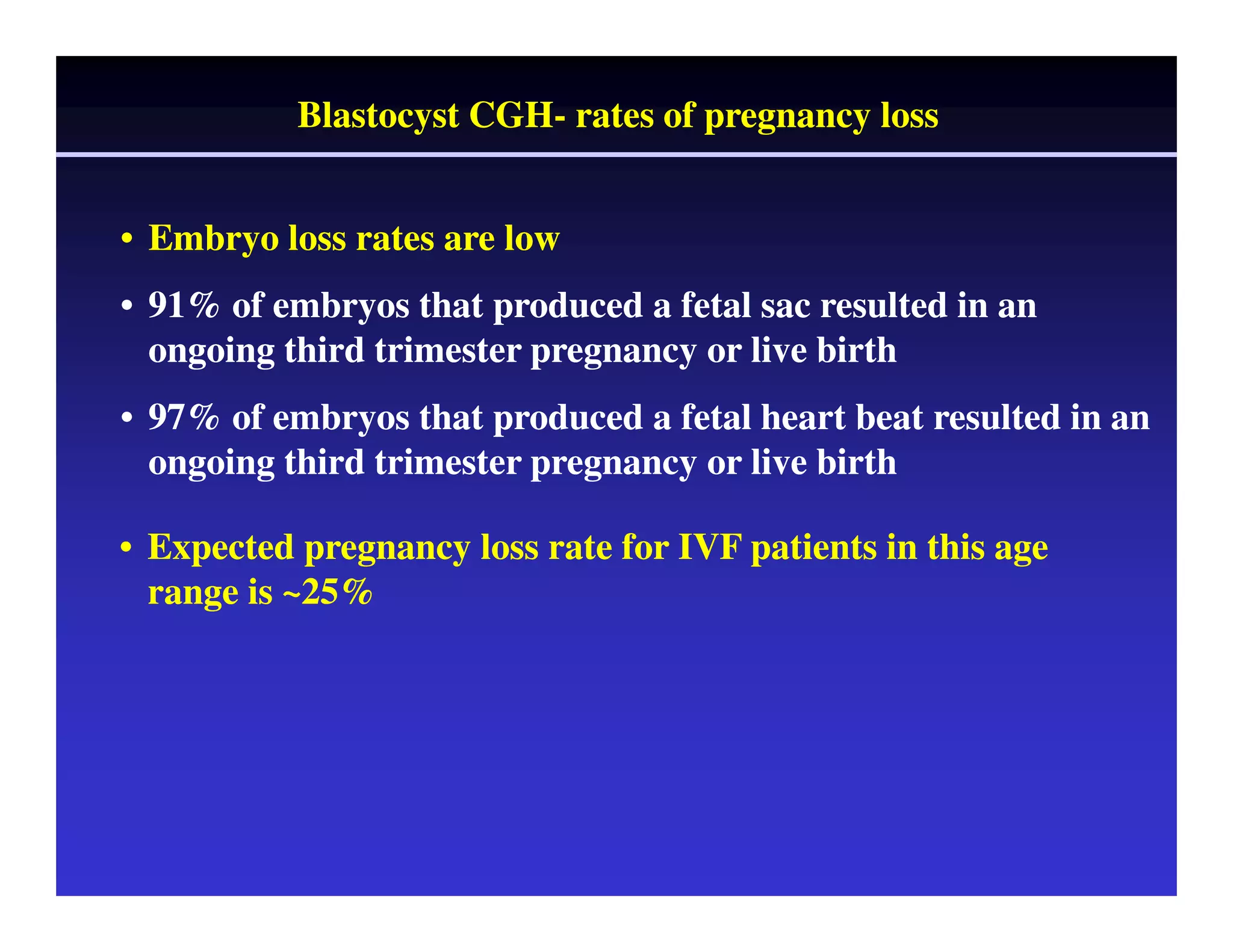
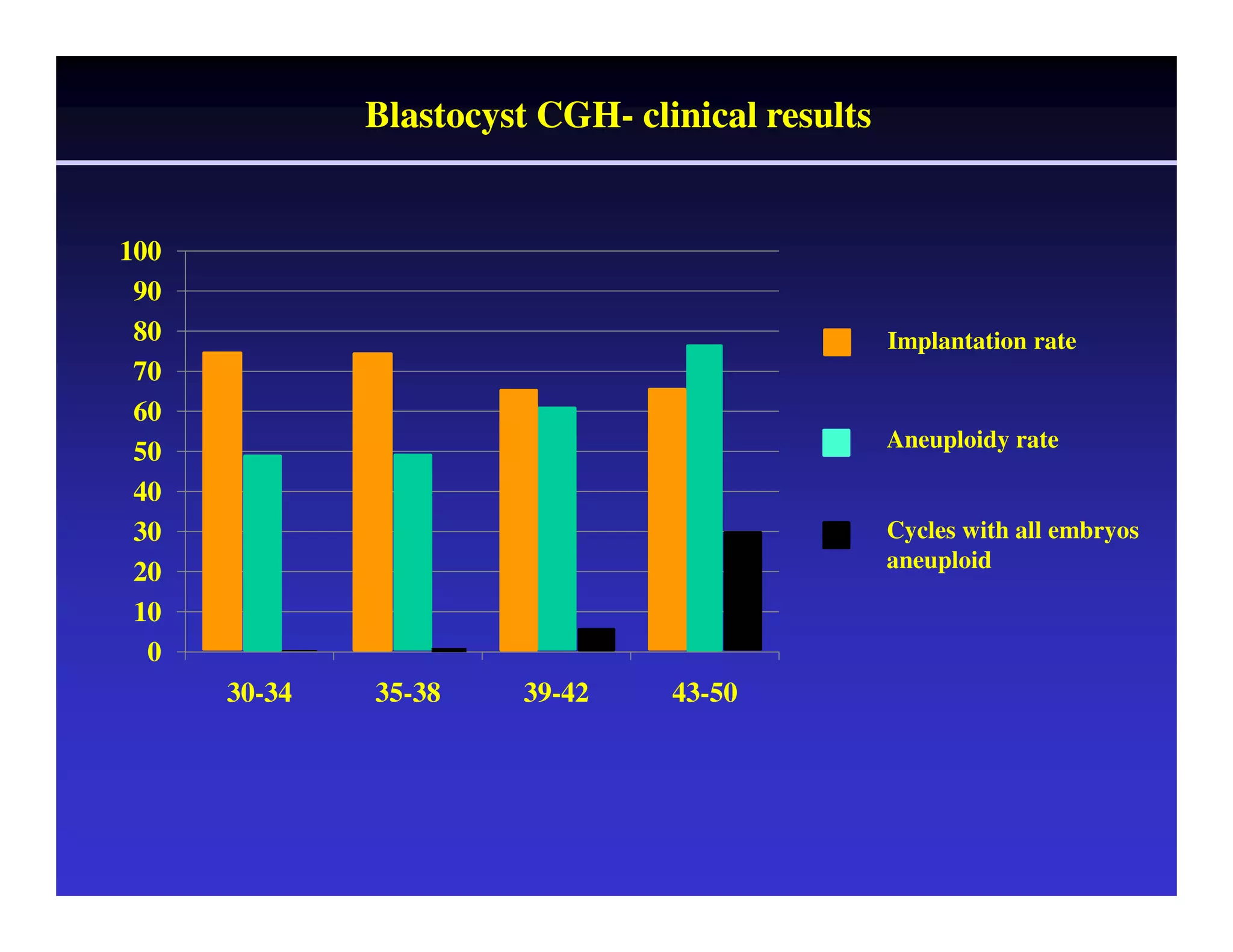
This document discusses chromosome screening and preimplantation genetic screening (PGS). It notes that aneuploidy increases significantly with maternal age and is a major cause of IVF failure and miscarriage. While PGS aims to increase implantation and pregnancy rates by screening embryos for aneuploidies, studies have shown mixed results. The document outlines various problems and limitations with past PGS studies that limit conclusions that can be drawn. It also discusses the potential benefits of PGS for recurrent implantation failure, previous trisomic conceptions, and recurrent pregnancy loss. Future developments discussed include using comparative genomic hybridization to screen all chromosomes and screening at the blastocyst stage.