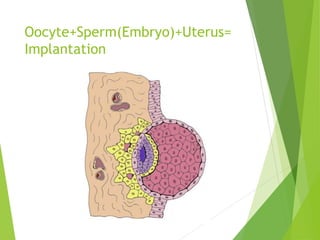
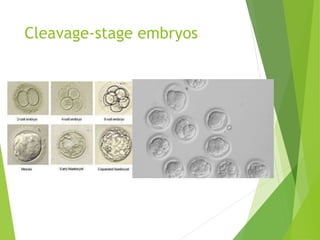
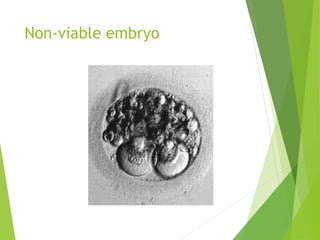
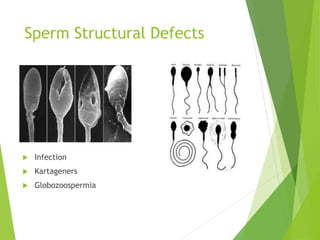
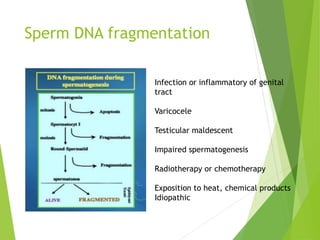
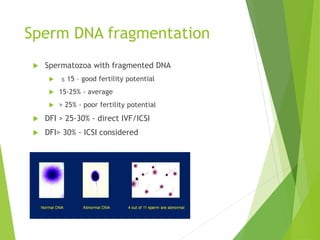
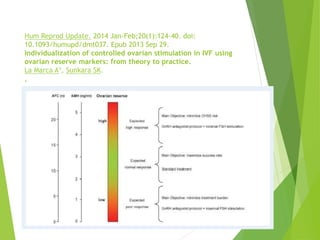
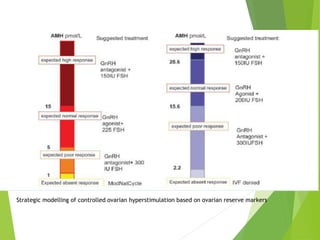
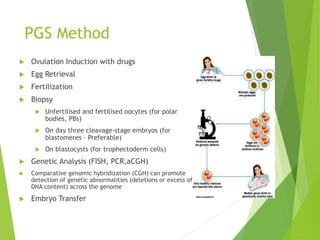
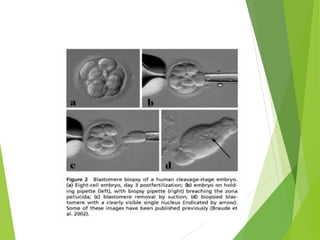
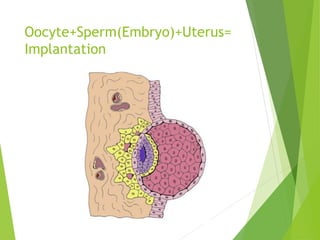
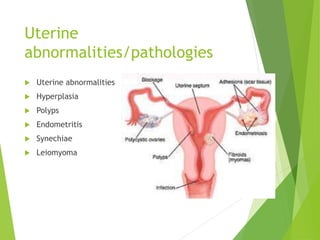
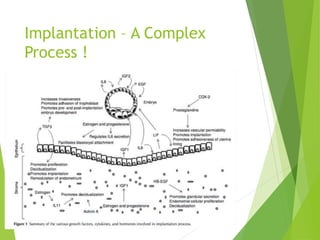
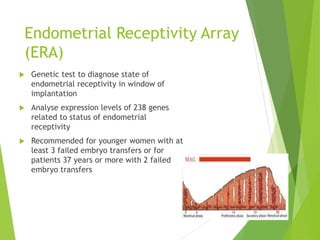
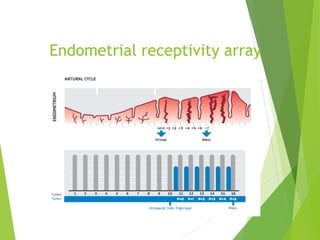
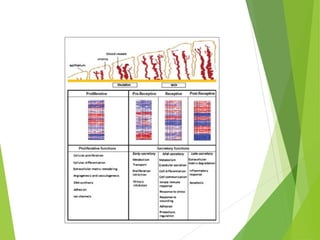
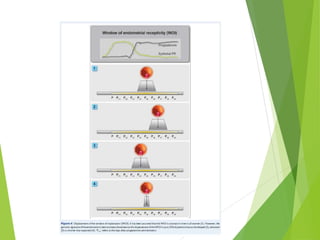
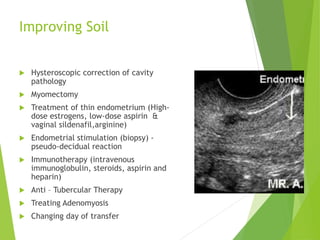
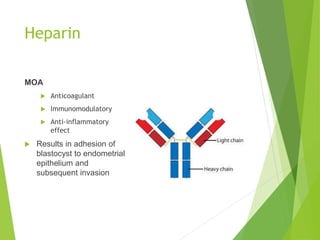
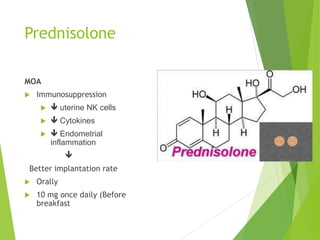
The document discusses recurrent implantation failure (RIF) after in vitro fertilization (IVF). RIF can be caused by factors related to the embryo, endometrium, or both. Embryo factors include poor egg or sperm quality, genetic abnormalities, or developmental issues. Endometrial factors involve uterine abnormalities, thin lining, altered receptors, or immunological incompatibility. Treatments aim to improve the embryo, such as by changing stimulation protocols, or the endometrium through hysteroscopic surgery, immunotherapy, or adjusting the transfer timing. Testing includes evaluating the embryos, endometrium, cavitary abnormalities, and immunological factors to guide personalized treatment strategies for RIF patients.