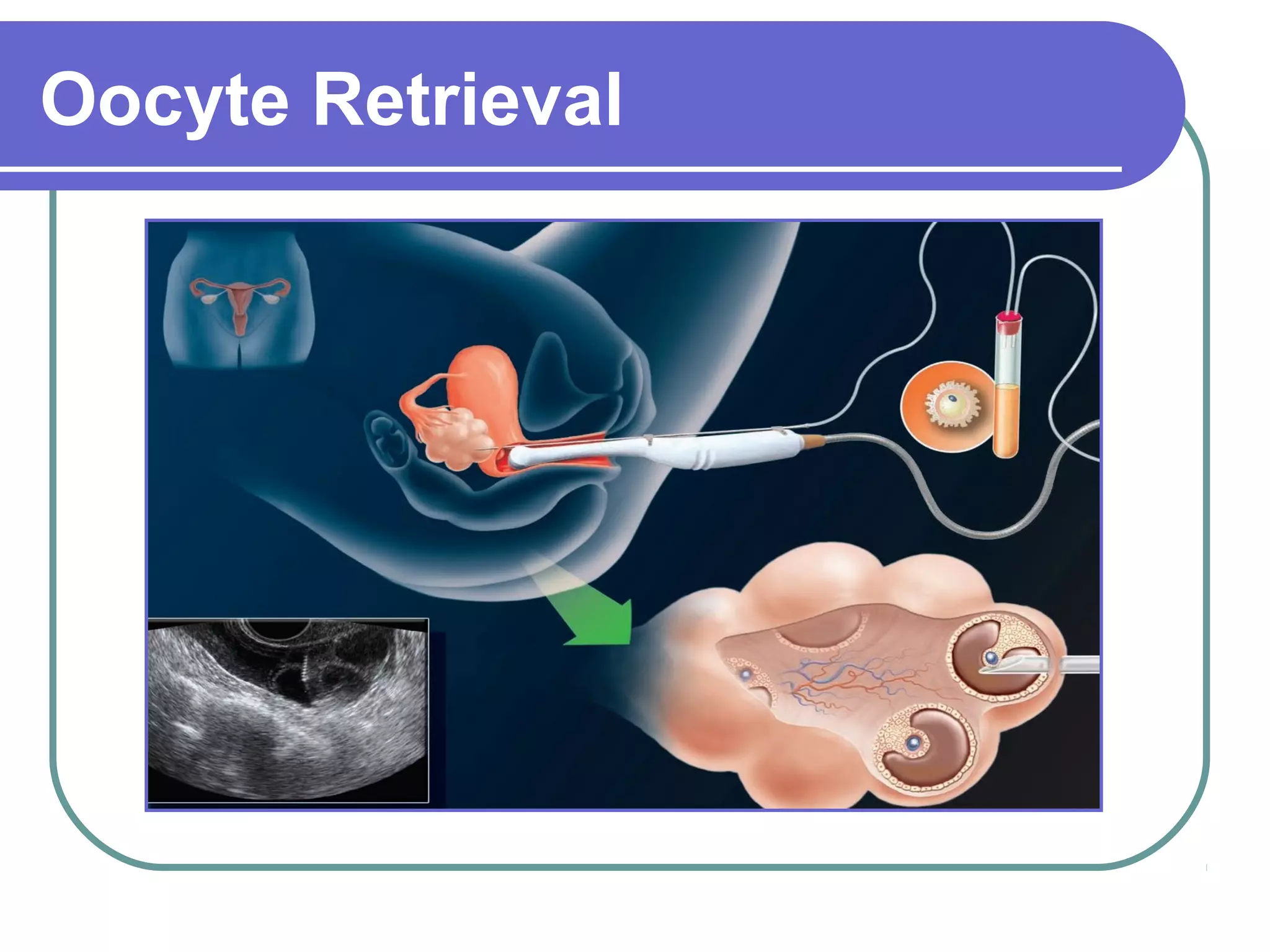
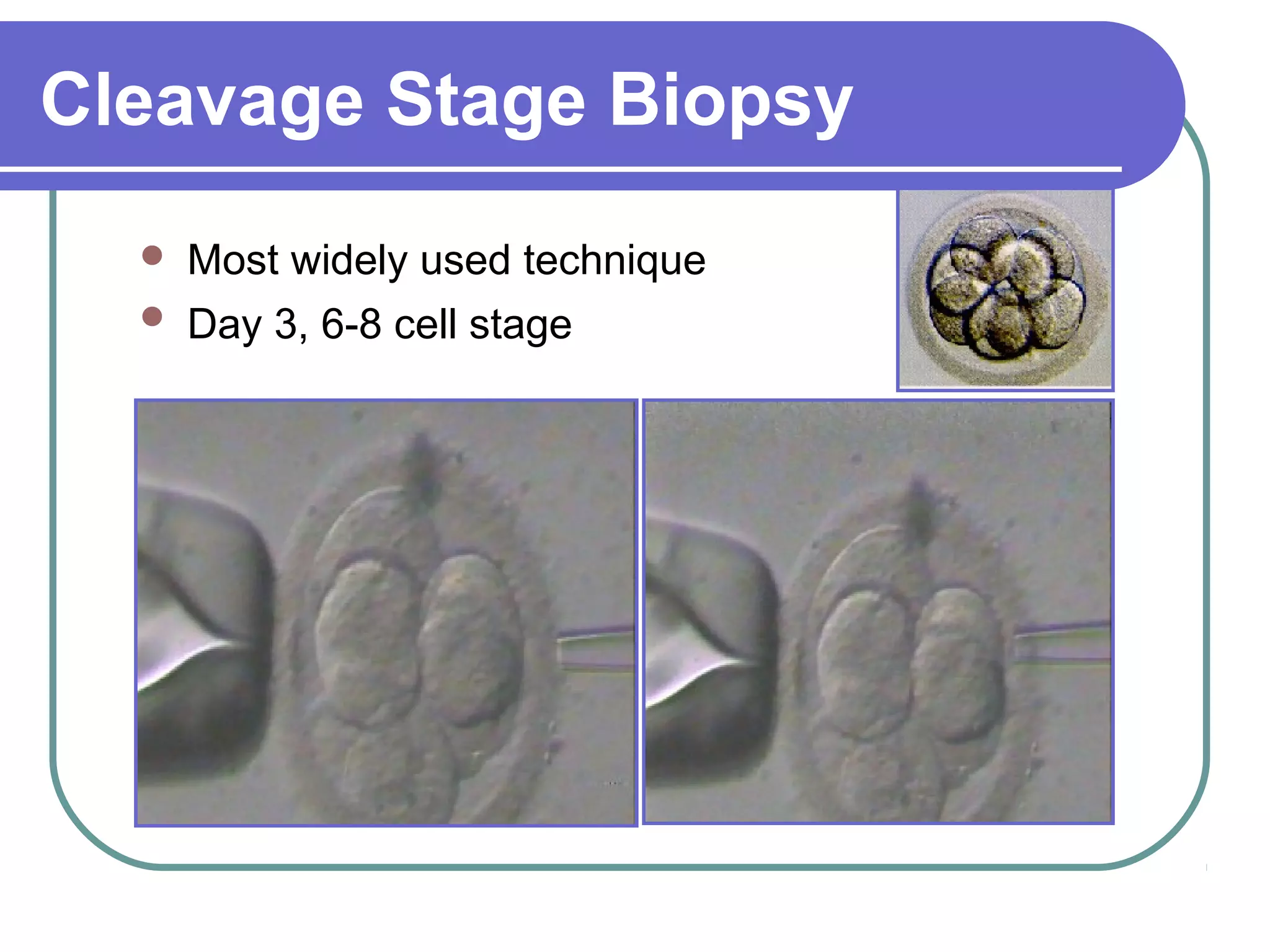
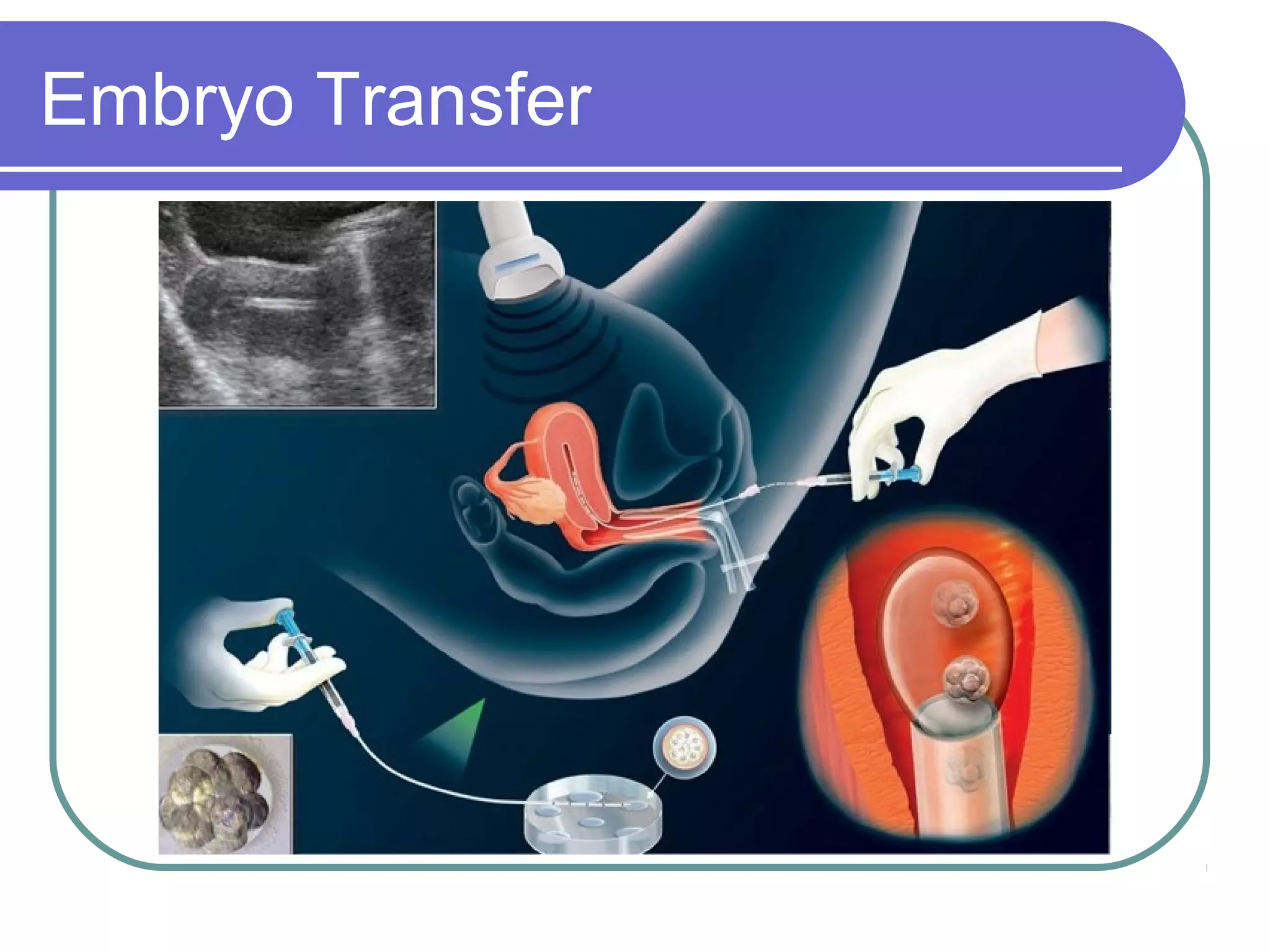
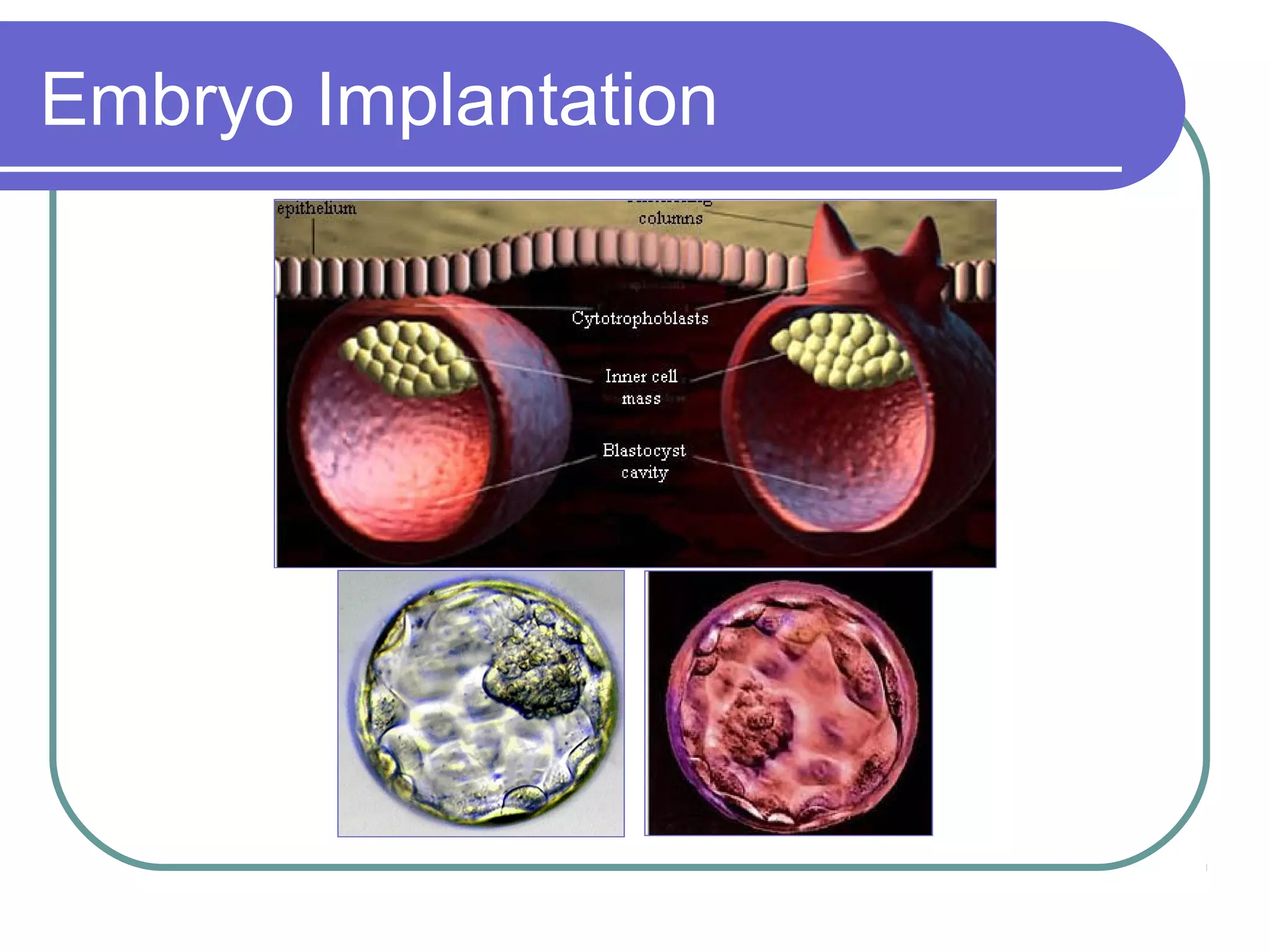
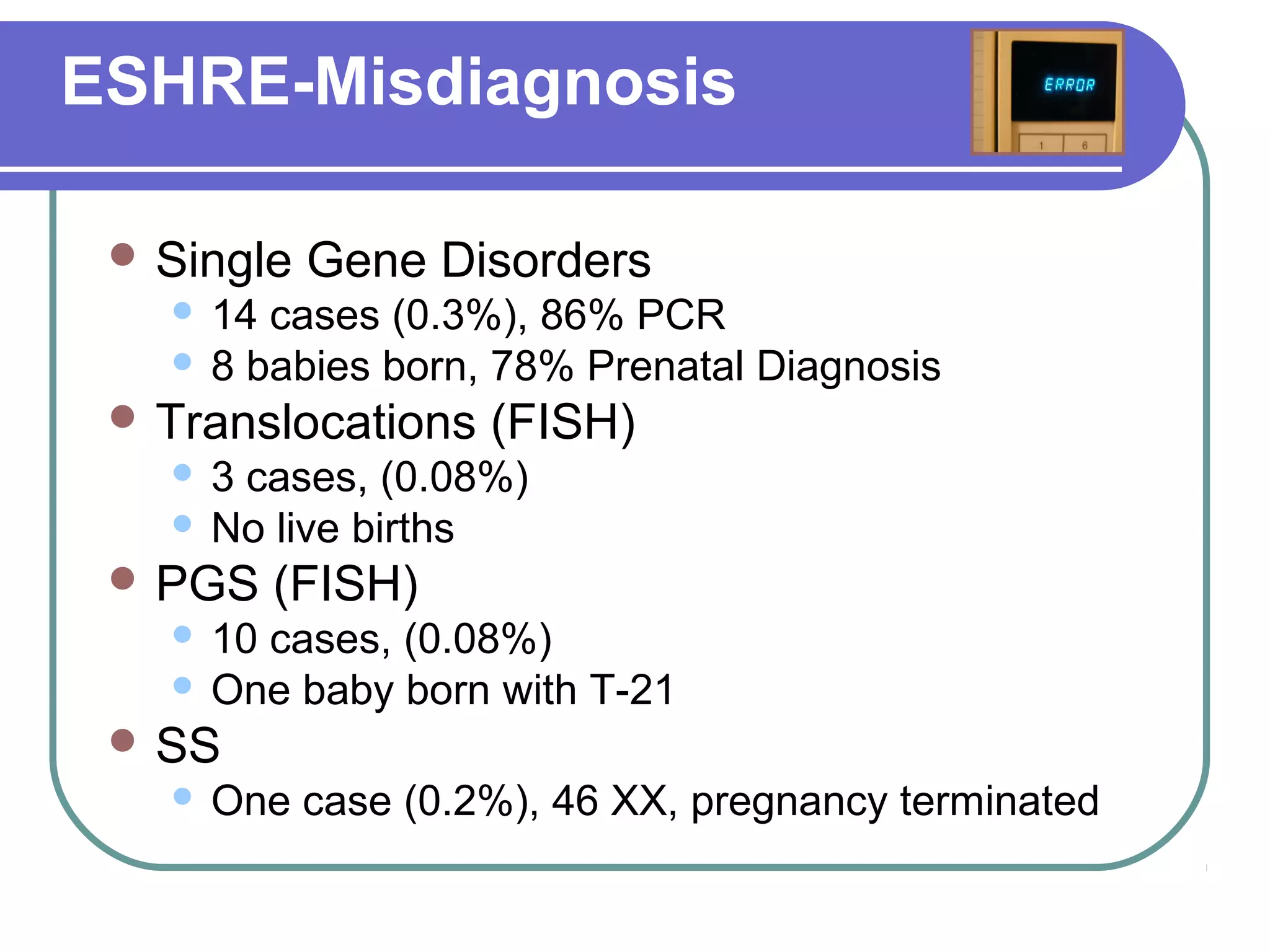
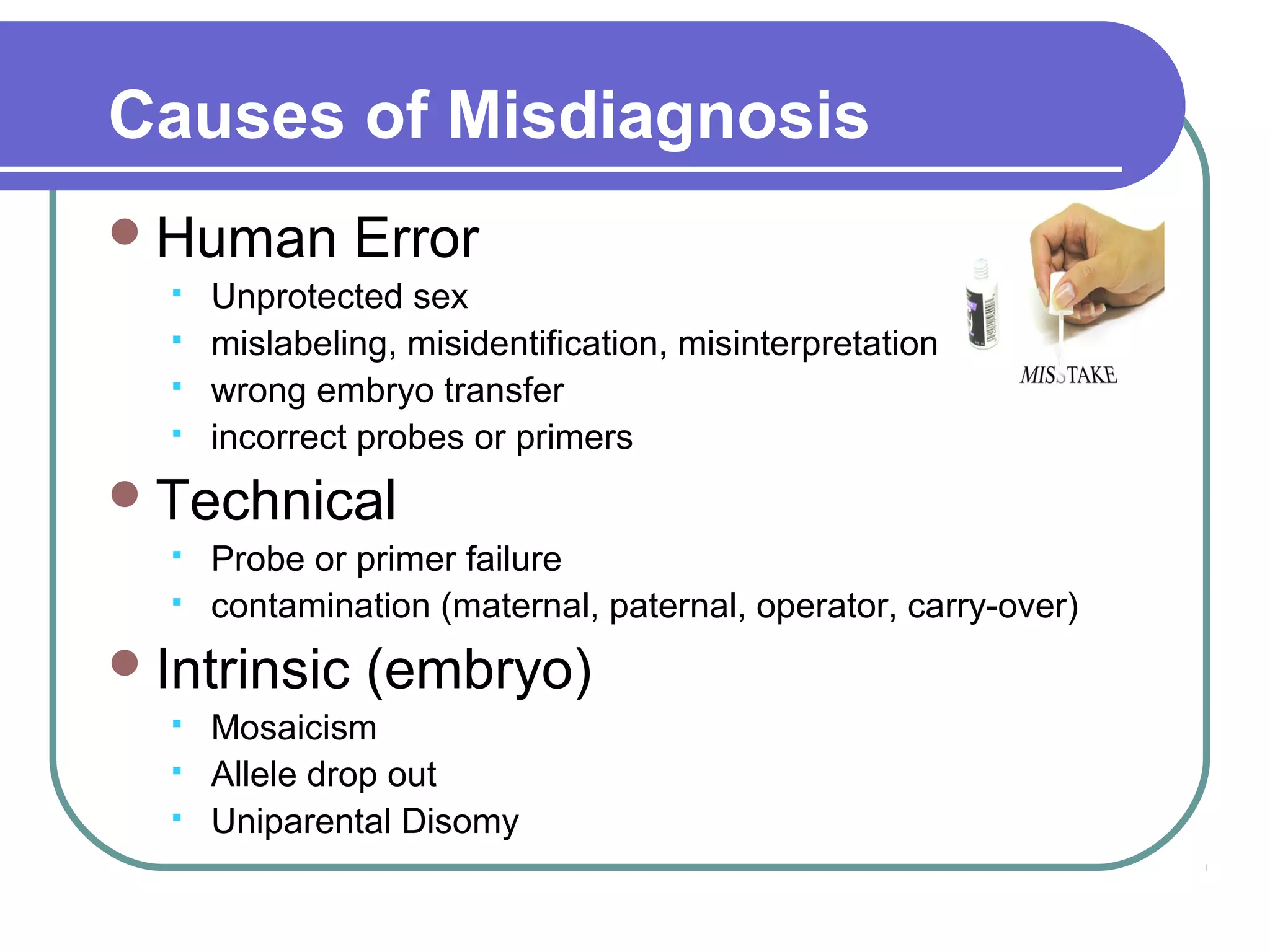
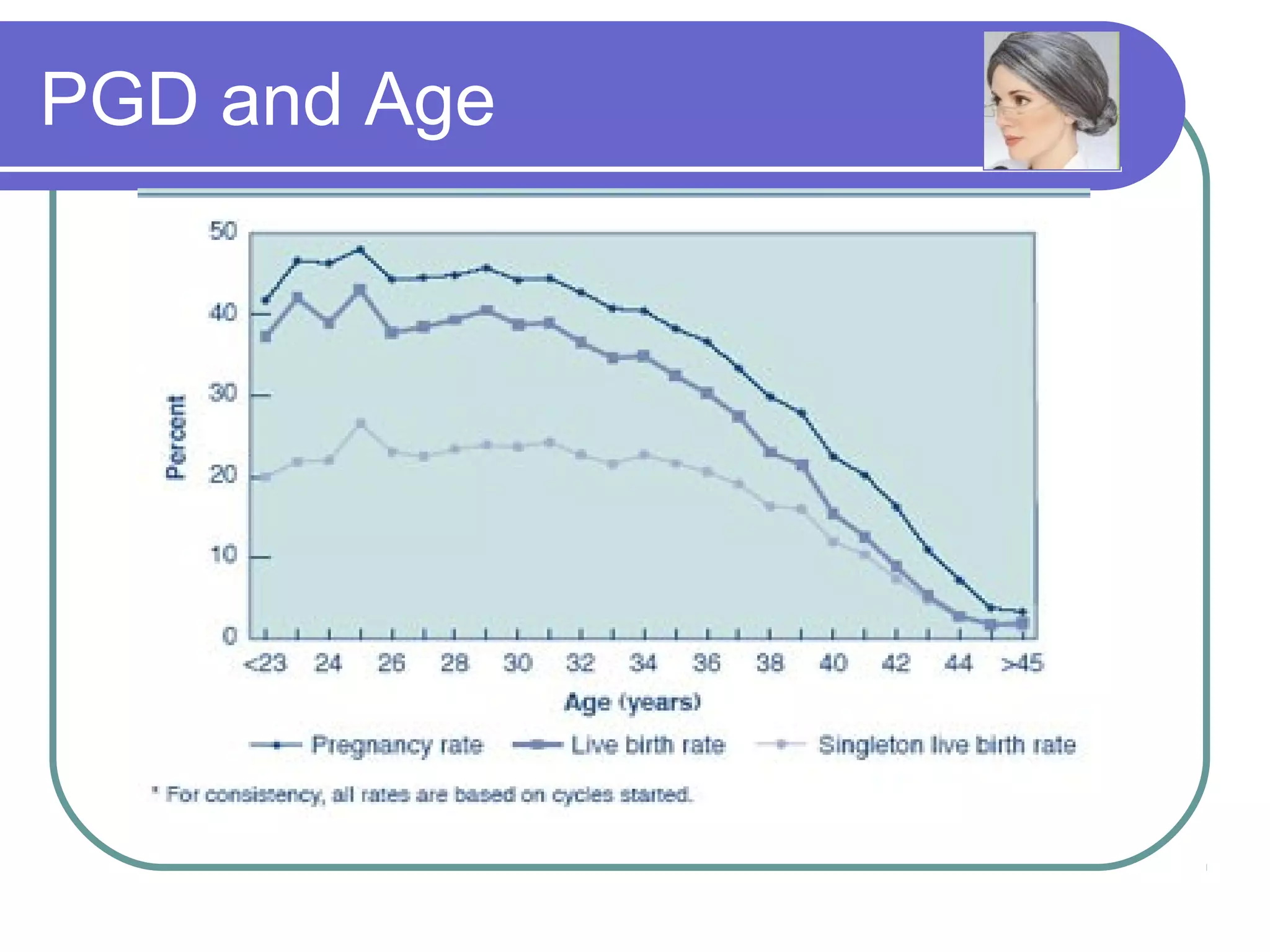
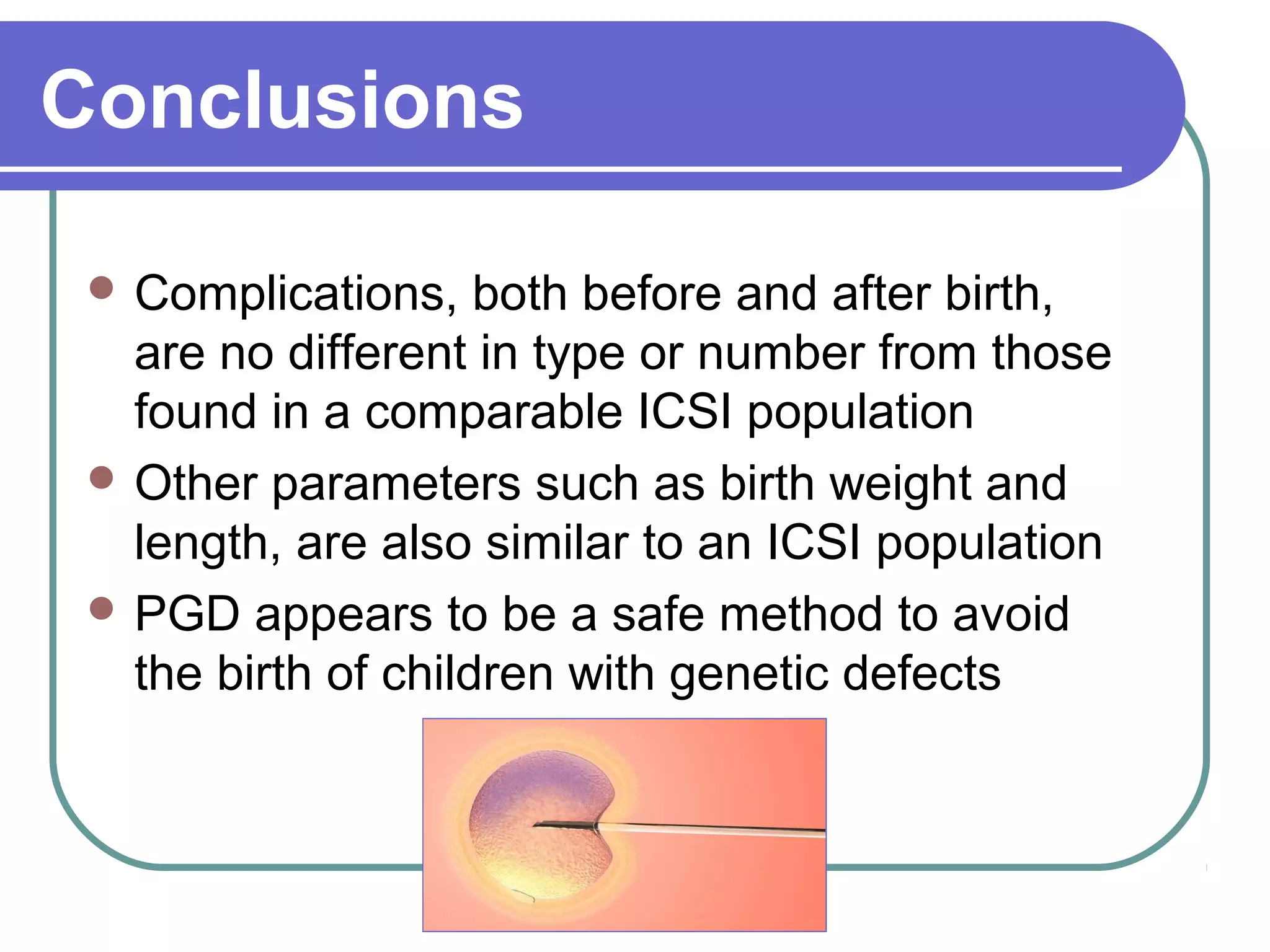
This document discusses preimplantation genetic diagnosis (PGD), which involves biopsy of a single cell from each embryo followed by genetic analysis to identify normal embryos for implantation. PGD is offered to couples at risk of passing on genetic disorders, chromosomal issues, or with recurrent pregnancy loss. The process involves ovarian stimulation, egg retrieval, fertilization, embryo biopsy on day 3, genetic analysis, and embryo transfer. Common indications for PGD include single gene disorders, translocations, aneuploidy screening, and HLA matching. While mistakes can occur, studies show delivery outcomes and malformation rates are similar to ICSI. PGD has allowed many to have healthy children who would otherwise be at high risk of genetic conditions.