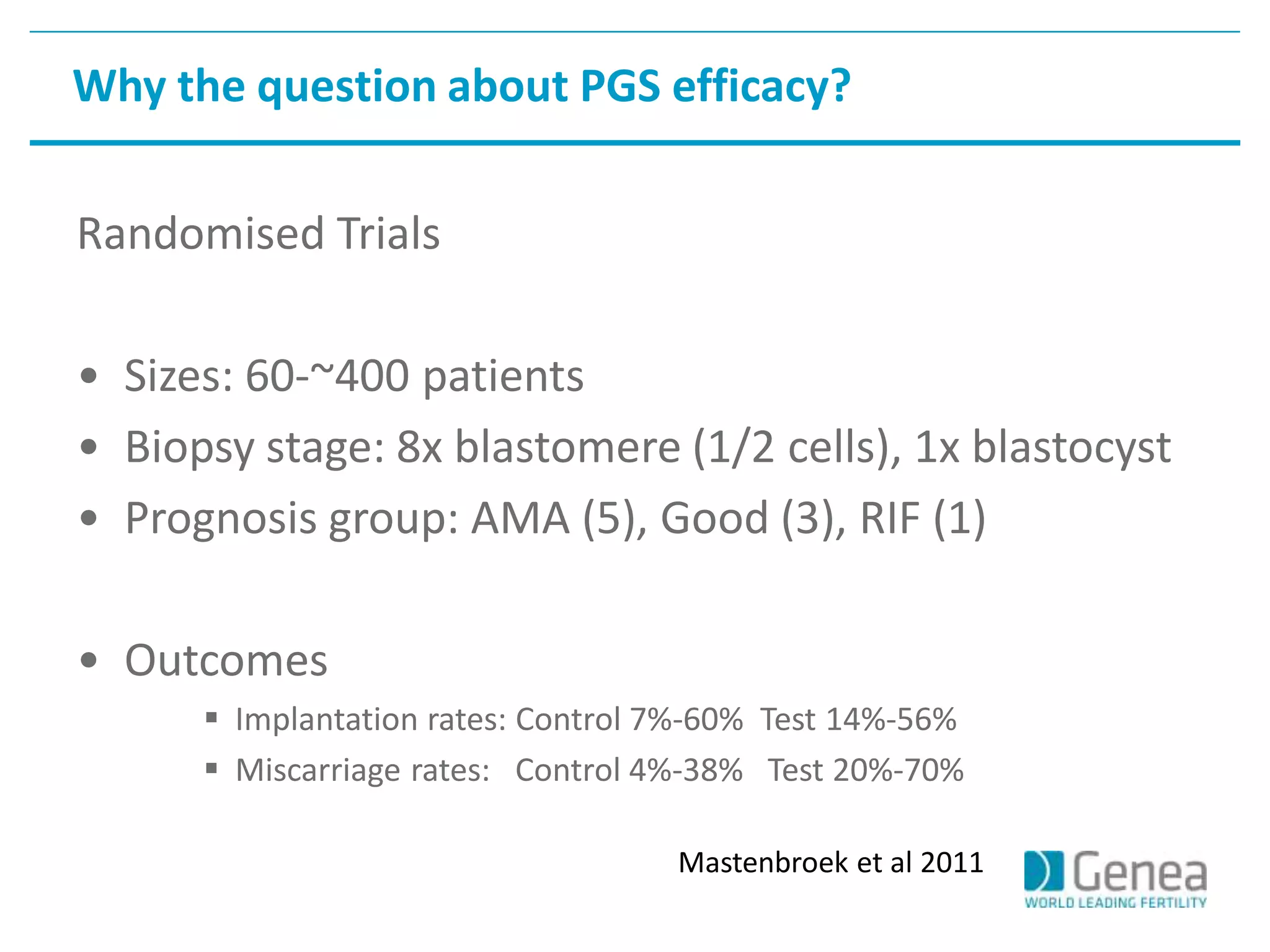
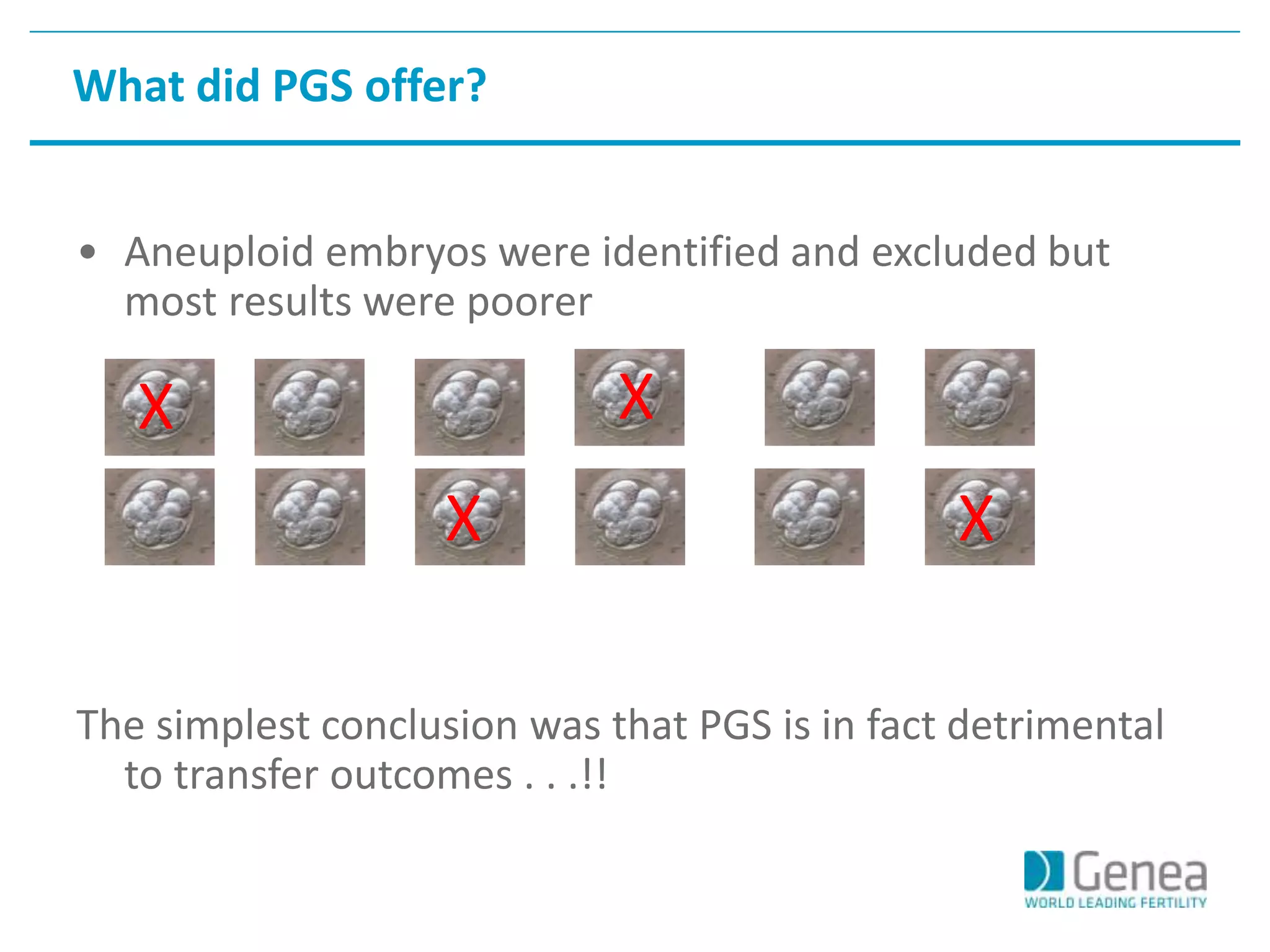
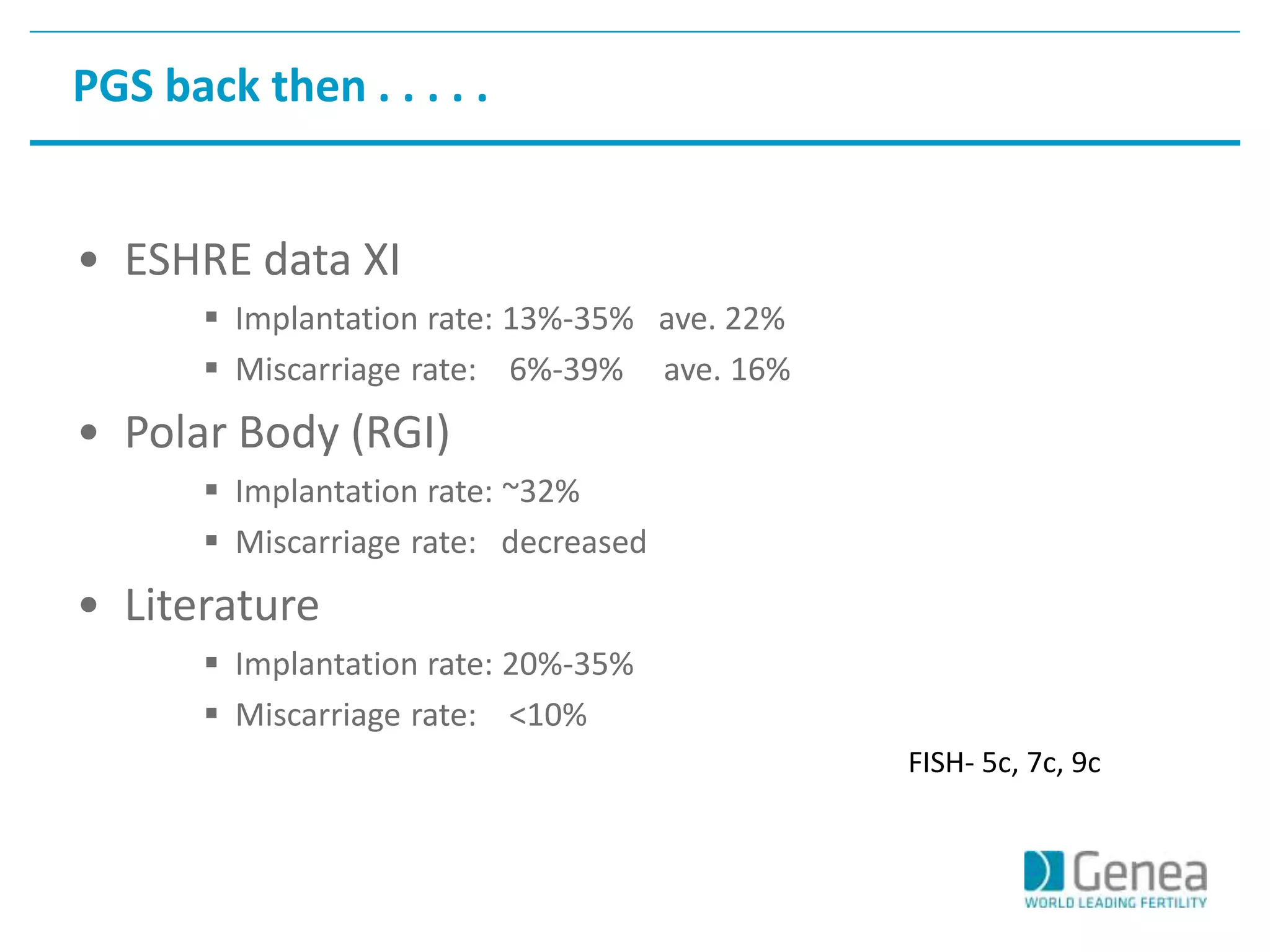
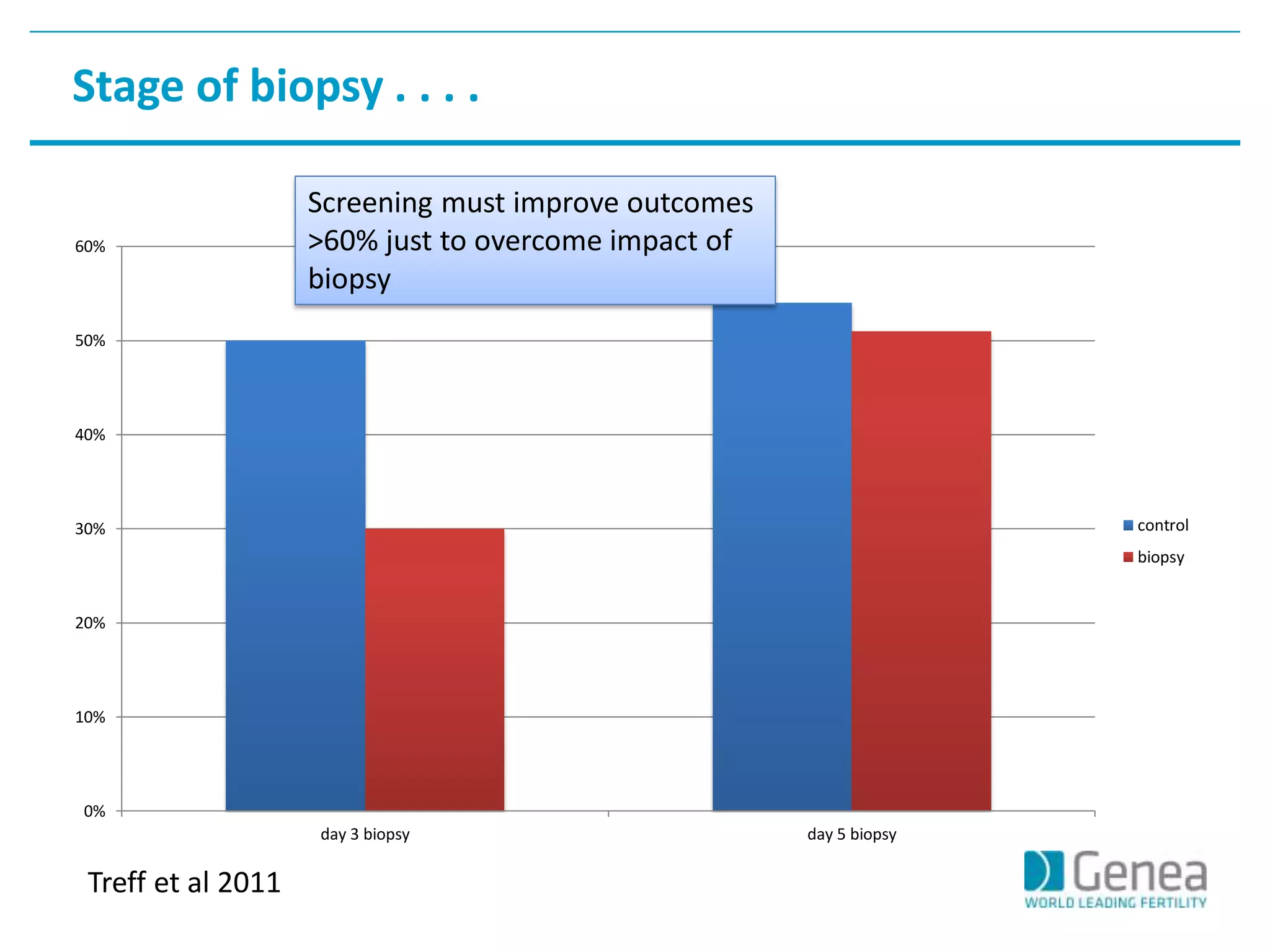
This document discusses evaluating the success of preimplantation genetic screening (PGS). It notes that early randomized trials of PGS found poorer outcomes compared to controls, likely due to technical limitations at the time like poor biopsy techniques and limited screening. More recent studies using day 5 biopsy and comprehensive chromosome screening are finding improved implantation and pregnancy rates with PGS. However, for PGS to be successful, all steps from egg retrieval to embryo transfer must be optimized. Outcomes also depend on patient factors, and PGS may not help all clinics or patients. The key measures of success are changes in implantation and pregnancy rates that exceed expected outcomes without PGS.