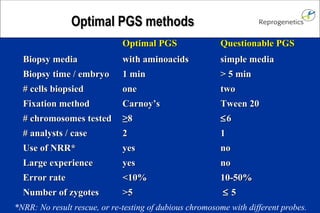
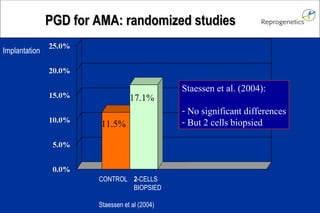
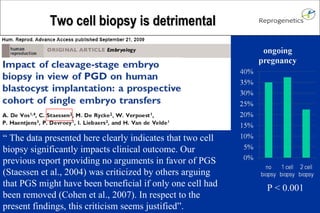
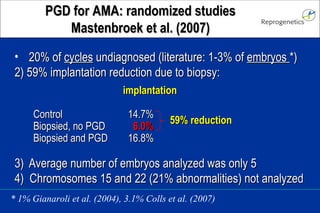
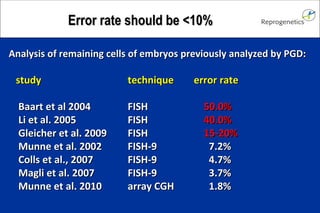
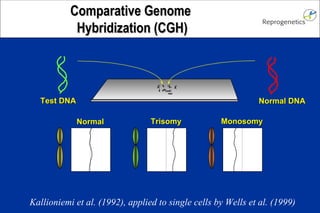
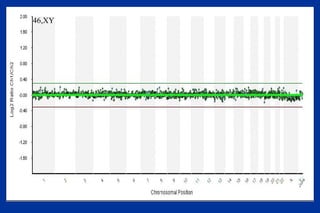
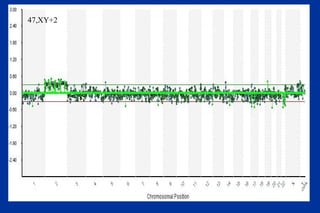
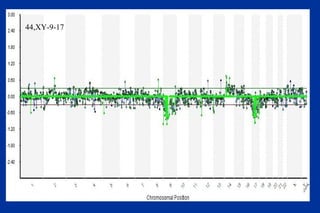
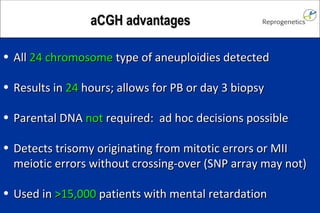
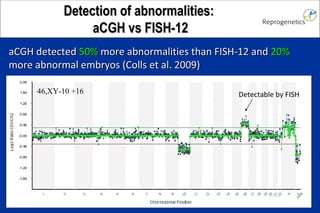
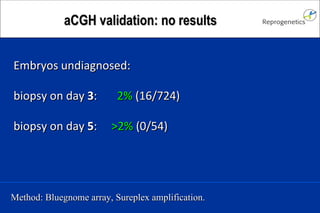
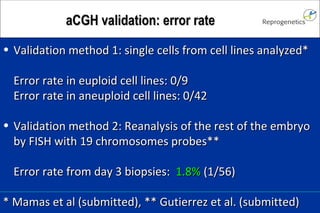
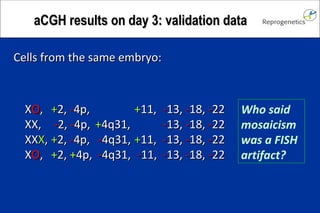
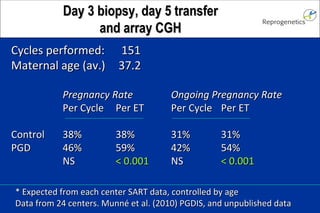
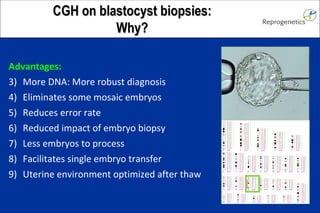
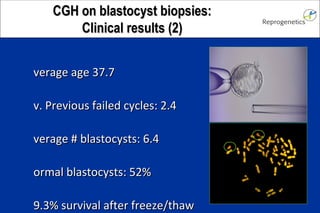
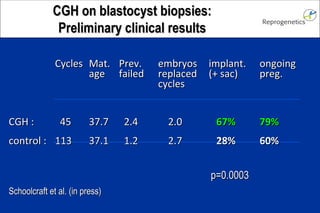
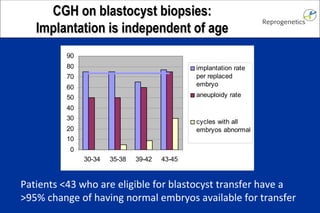
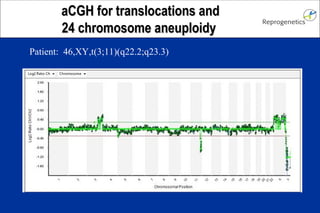
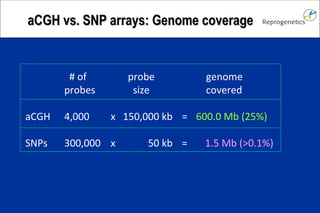
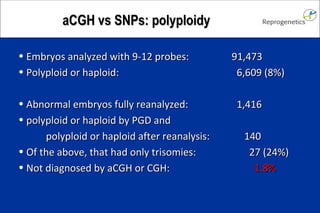
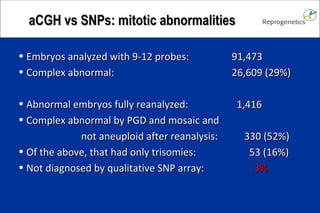
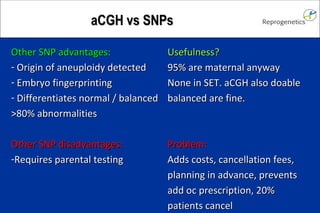
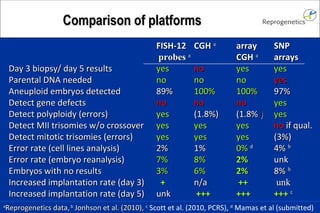
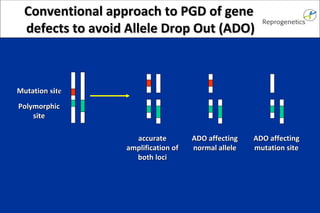
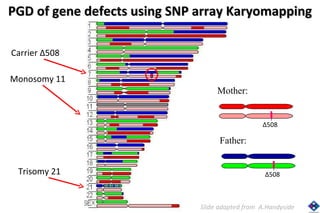
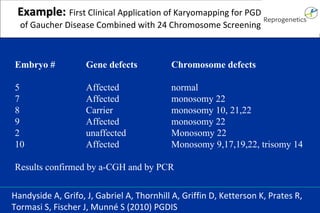
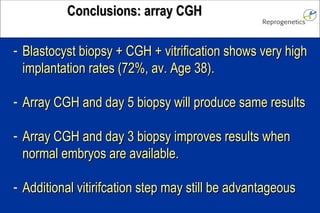
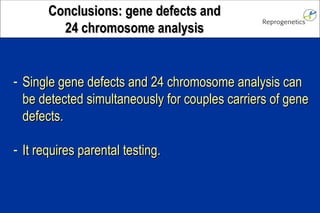
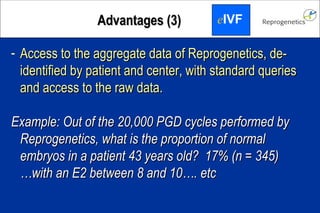
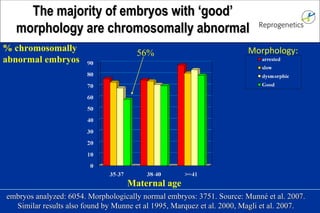
The document discusses preimplantation genetic diagnosis (PGD) and its services, revealing significant data about chromosomal abnormalities in embryos and the efficacy of different biopsy methods and analytical techniques. It highlights the benefits and limitations of various PGD methods, particularly in relation to advanced maternal age, and examines the impact of aneuploidy on IVF success rates. The study suggests that improved methods like array comparative genome hybridization (aCGH) provide better diagnostic accuracy for chromosomal abnormalities than traditional techniques.







![592 embryos found abnormal by PGD were reanalyzed and found to be: normal 13 mosaic <49% abnormal 27 mosaic 50-99% abnormal 124 mosaic 100% abnormal 297 homogeneously abnormal 131 Colls et al. (2007) Mosaicism produces <7% misdiagnosis 6.8% 1[13]1[16]2[18]2[21]1[22] 2[13]1[16]2[18]2[21]2[22] 1[13]1[16]2[18]2[21]1[22] 1[16] 2[13]2[16]2[18]2[21]2[22] 2[13]1[16]2[18]1[21]1[22] 2[13]3[18]1[21]1[22] 3[13]1[16]2[18]1[21]3[22] 1[13]1[16]1[18]1[21]1[22] 3[13]1[16]2[18]1[21]3[22]](https://image.slidesharecdn.com/munnearraycghupdate201005-12736039844094-phpapp02/85/Munne-Array-Cgh-Update-2010-05-10-320.jpg)