Embed presentation
Download to read offline




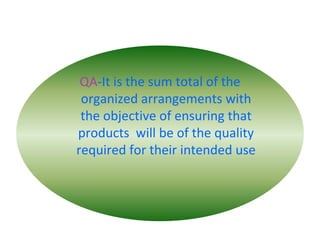



GMP in the pharmaceutical industry ensures consistent high quality production of medicines. GMP represents creating, implementing, and sustaining a culture of quality compliance. It is part of quality assurance aimed at ensuring products are consistently manufactured to the required quality for their intended use. The objectives of GMP are to understand key concepts of quality assurance, quality control, and GMP regulations as well as basic requirements for each GMP element. Medicines must be of the highest strength, purity, and quality since they are potent tools used for treating human beings.