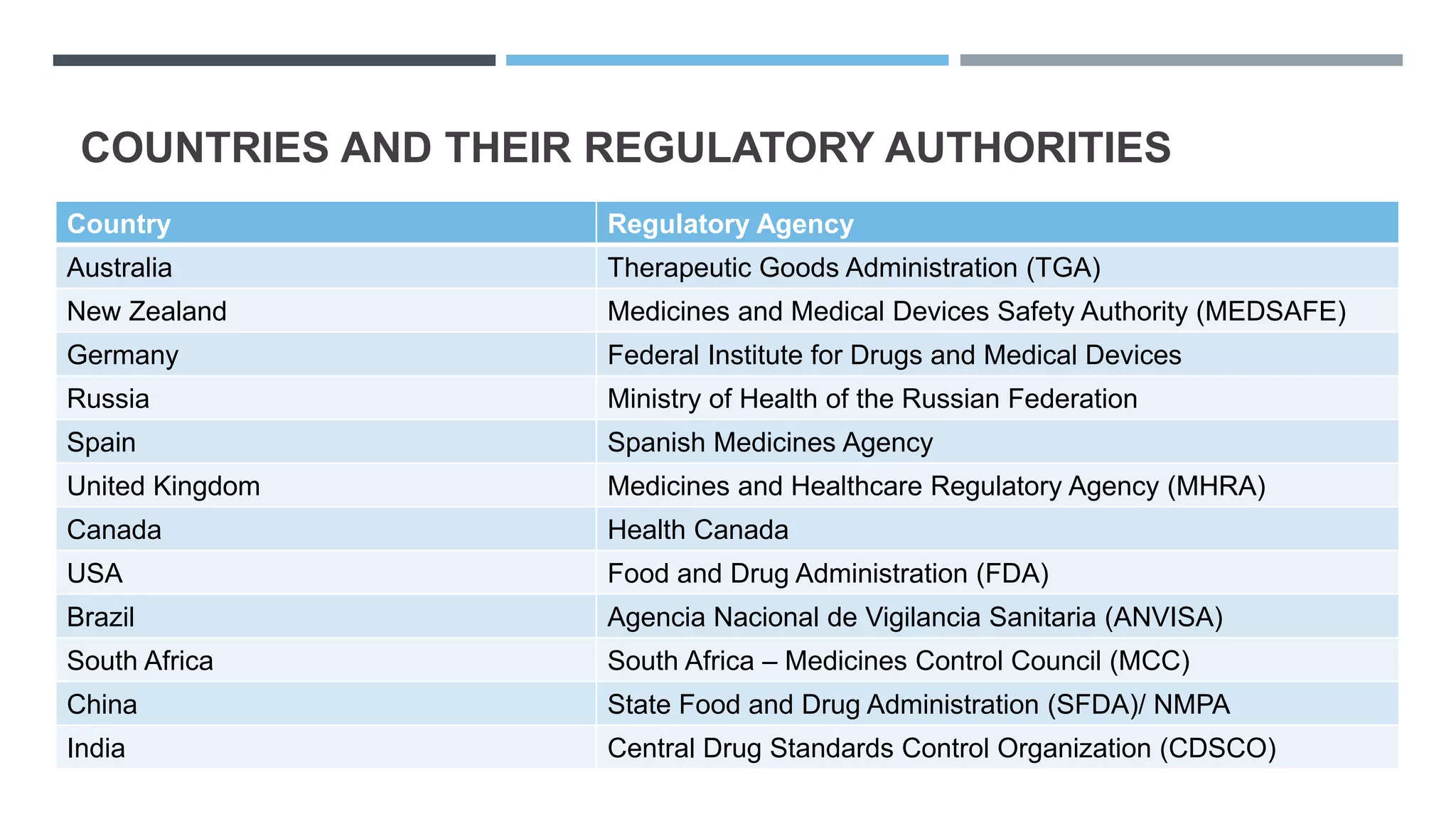
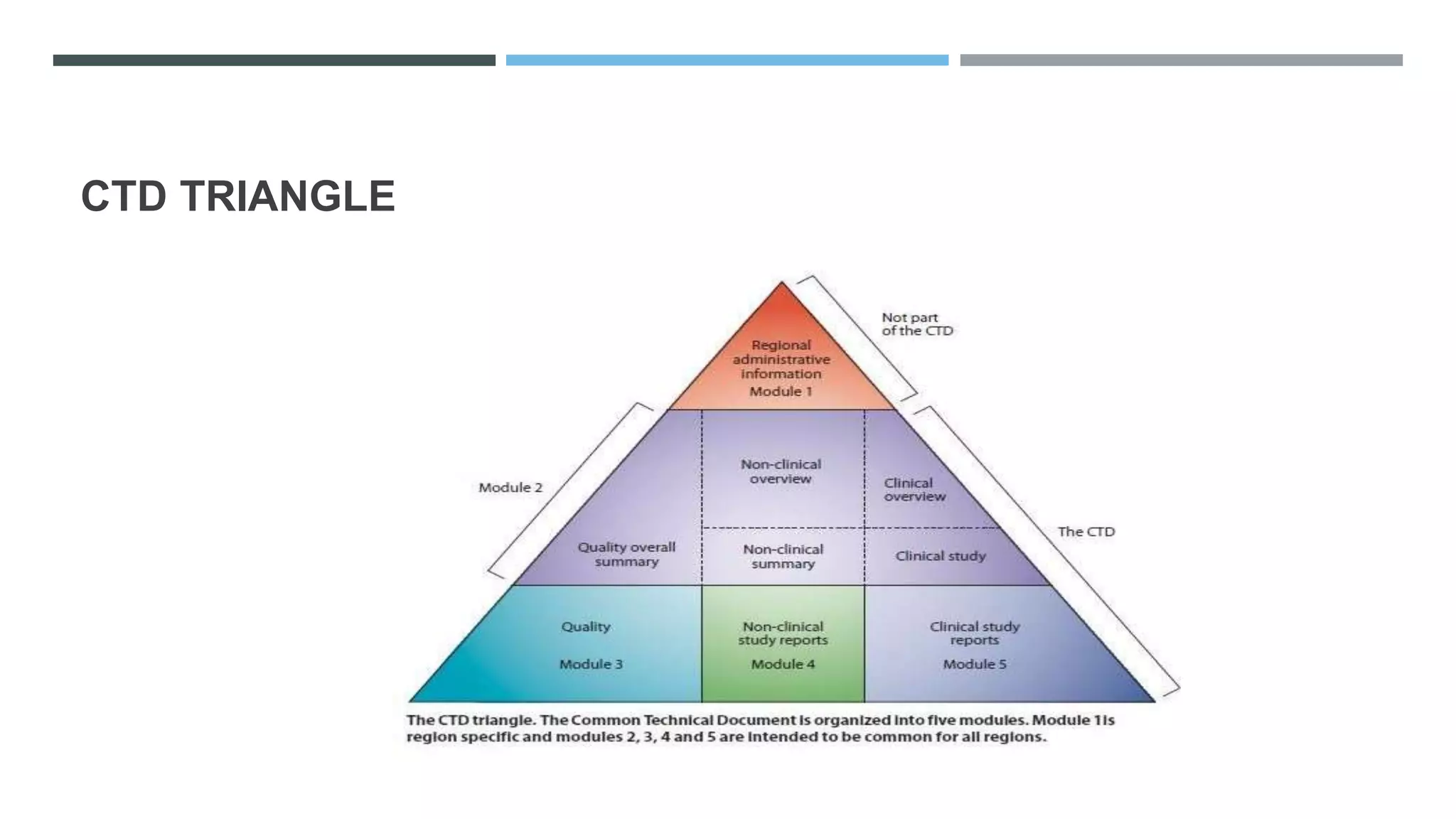
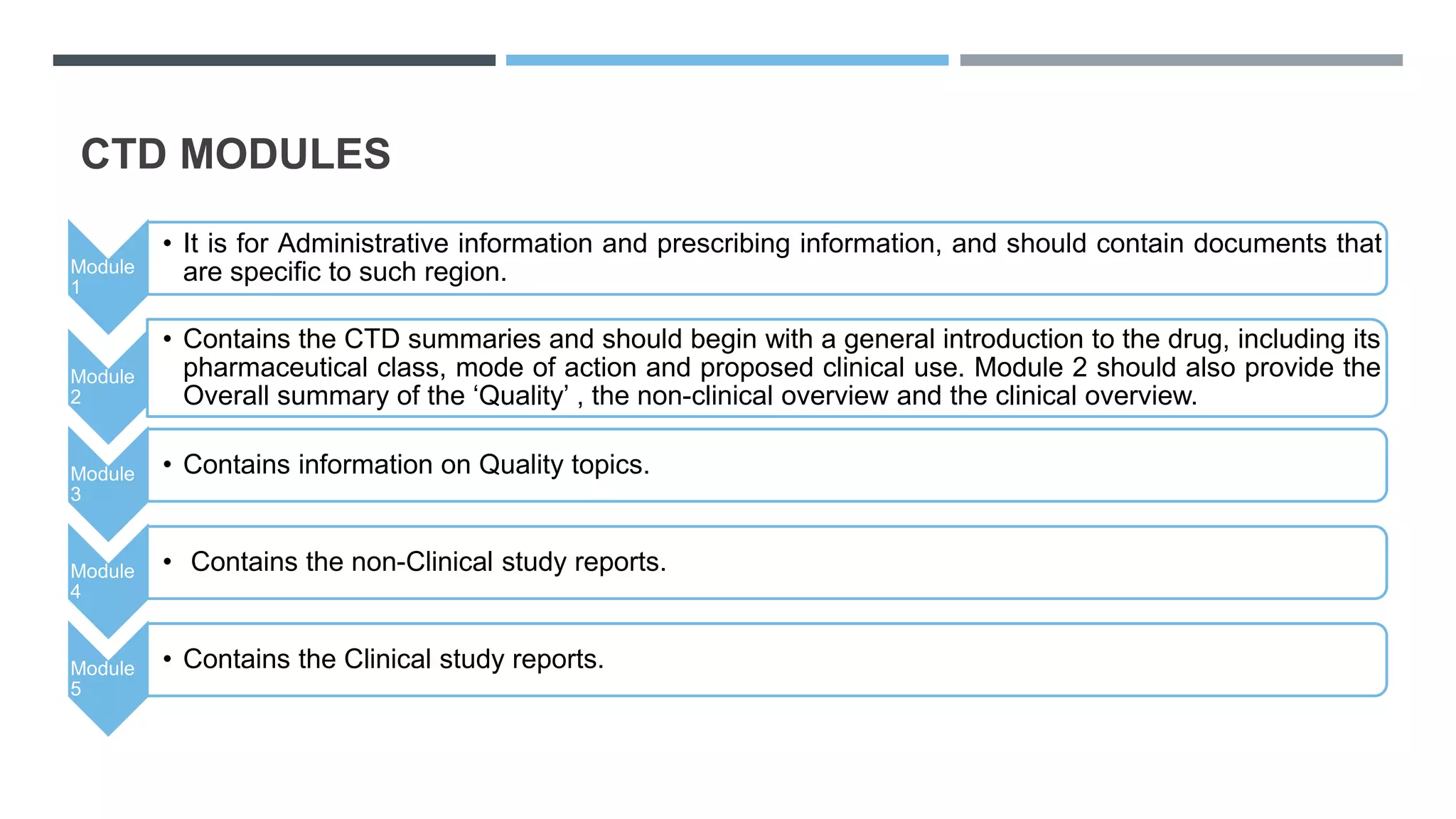
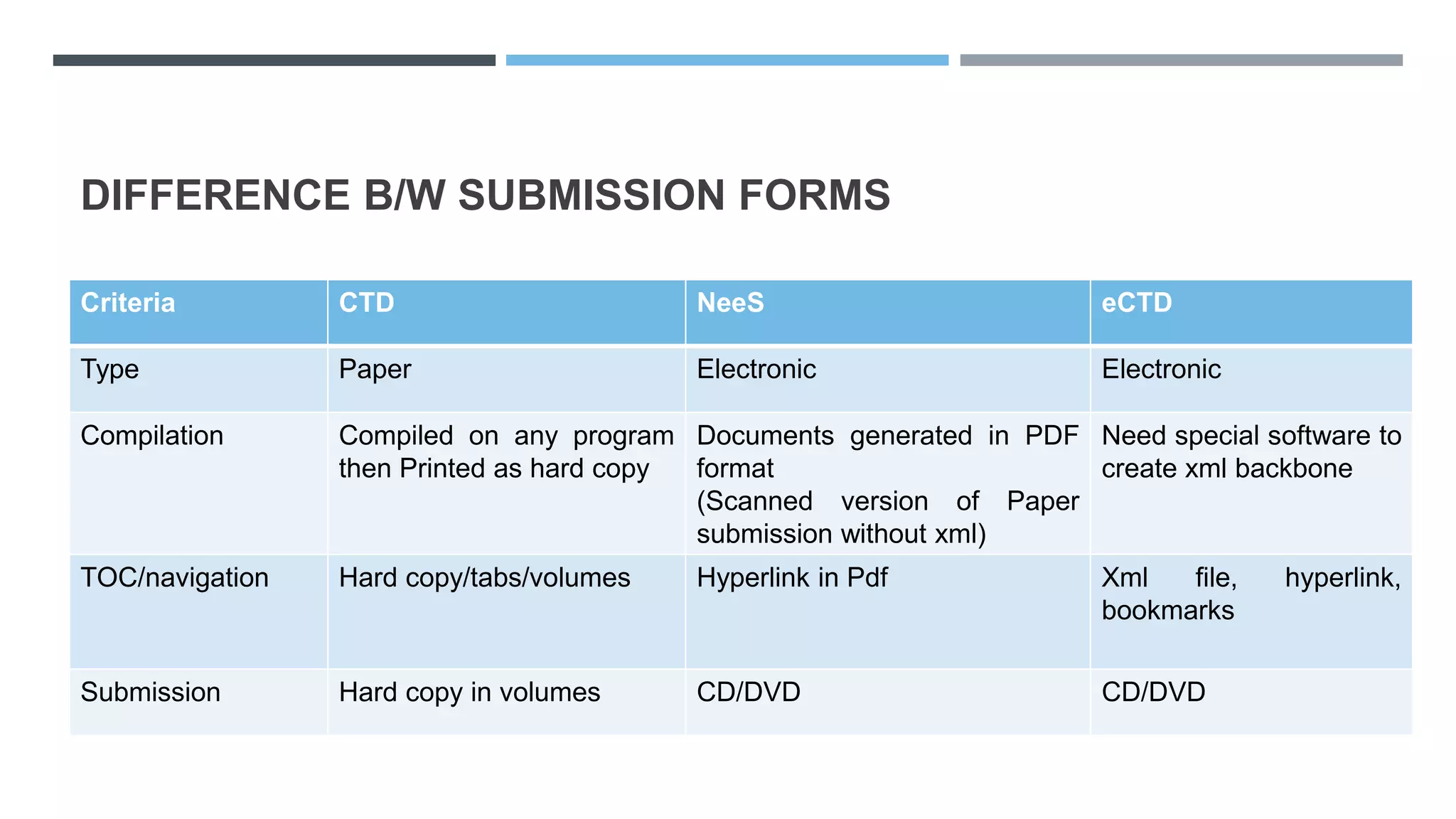
This document provides an overview of regulatory dossier preparation and submission using the Common Technical Document (CTD) format. It defines a regulatory dossier and describes the two main types: ICH-CTD and ASEAN CTD. It lists several countries and their respective regulatory authorities. The CTD is explained as a joint format maintained by regulatory agencies in Europe, Japan, and the US. The five CTD modules are outlined which contain administrative, quality, non-clinical, and clinical information. Finally, it compares paper, NeeS, and eCTD submission formats in terms of features like type, compilation, and submission method.