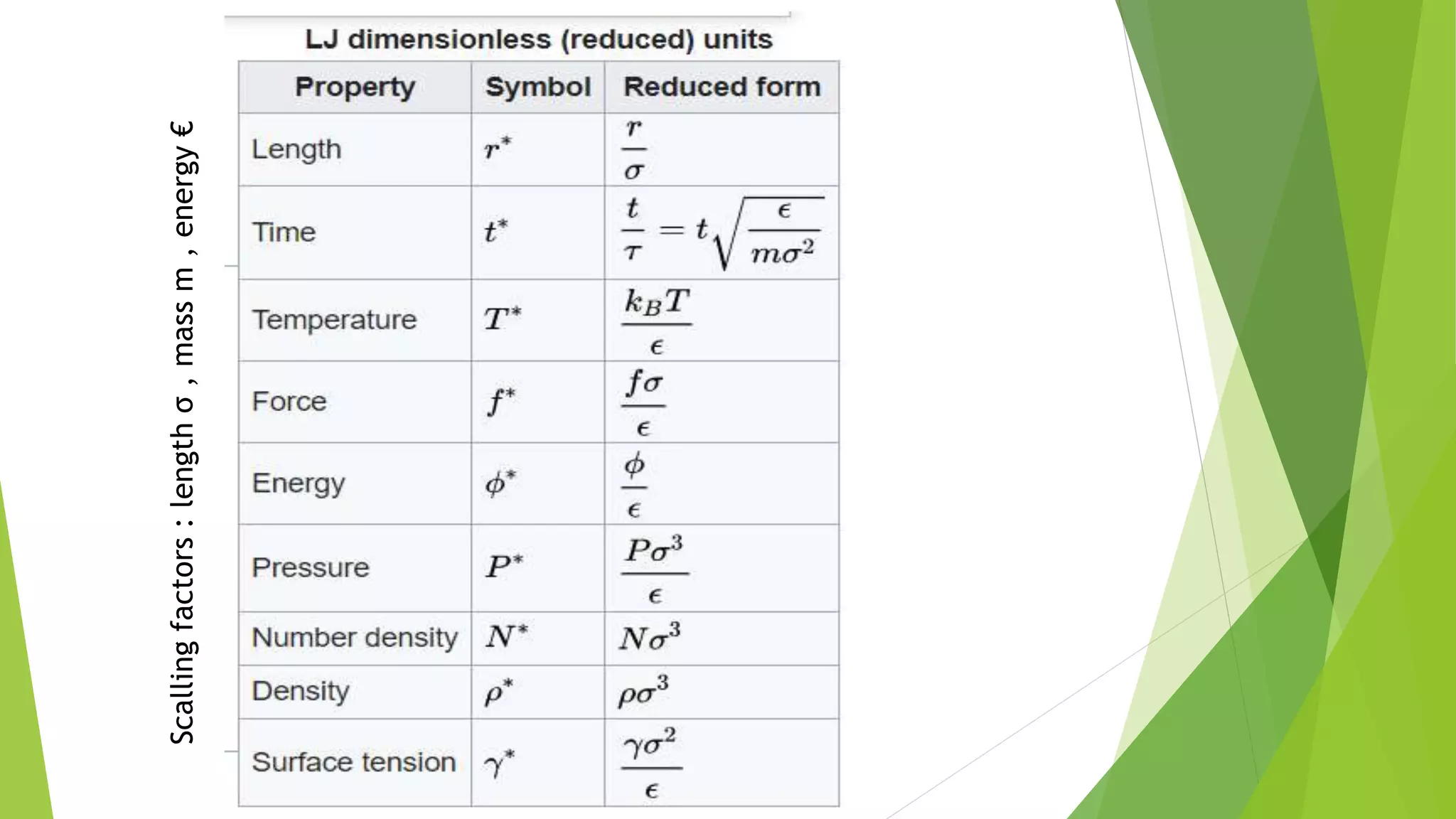
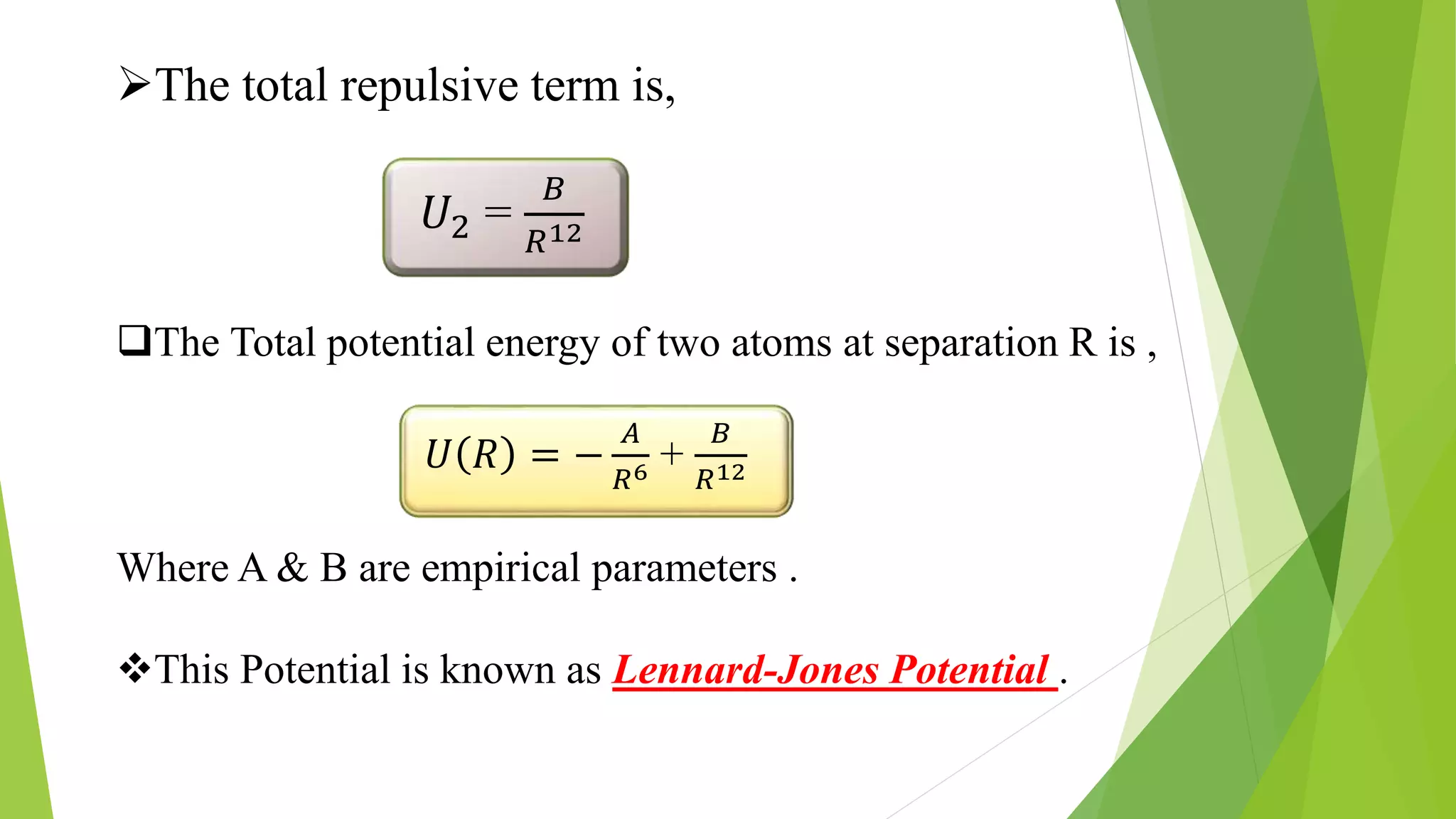
The document discusses the Lennard-Jones potential model for describing interactions between atoms or molecules. It consists of two terms: an attractive dispersion term proportional to 1/R6 representing van der Waals forces, and a repulsive term proportional to 1/R12 representing Pauli repulsion at short distances. The potential is commonly used to model properties of inert gas crystals and determine their lowest energy configurations. However, it has limitations as it oversimplifies real bonding and does not account for directionality or many-body effects.










![Curve of Lennard-Jones Potential
U(R)=4∈[ (σ/R)^12 – (σ/R)^6 ]
Where , A = 4∈σ^6 & B = 4∈σ^12](https://image.slidesharecdn.com/crystalsofinertgases-181026161451/75/Crystals-of-inert-gases-14-2048.jpg)