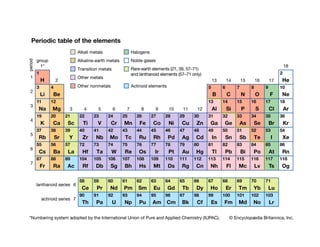
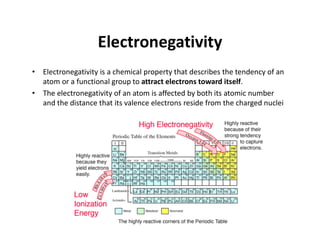
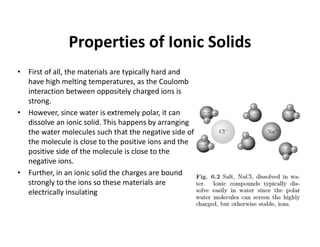
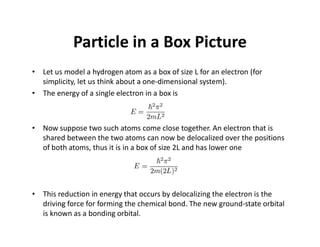
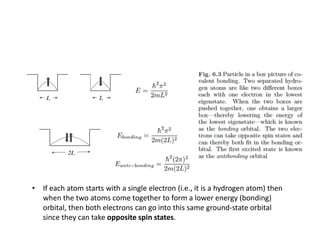
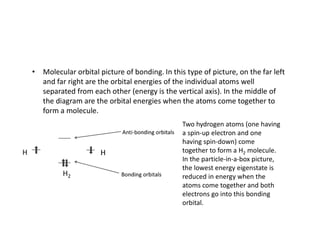
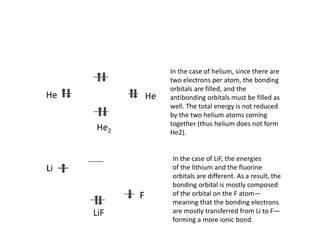
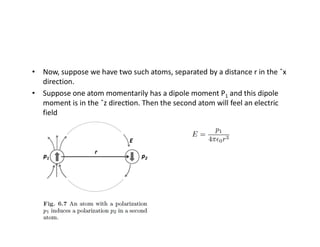
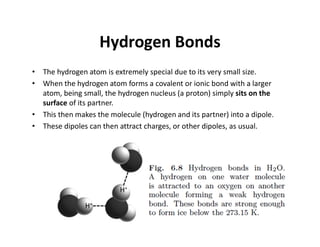
The document discusses the principles of solid state physics, focusing on chemical bonding, including ionic, covalent, Van der Waals, metallic, and hydrogen bonds. It explains key concepts such as electron affinity, ionization energy, and electronegativity, which influence how atoms bond together in solids. The text highlights the characteristics and properties of different types of bonds, detailing how these interactions affect the behavior of materials.