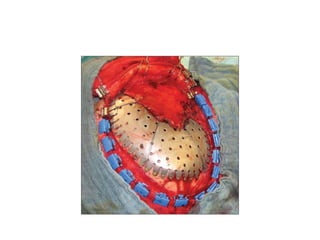
Cranioplasty is a surgical procedure to reconstruct and repair a defect in the skull. Some key points:
- The first documented cranioplasty used a piece of dog cranium to repair a defect in a Russian nobleman in the 17th century.
- Autologous bone is still considered the best graft material due to its biocompatibility and ability to integrate with native bone. Other common materials include methyl methacrylate, titanium, and hydroxyapatite.
- Indications for cranioplasty include protecting the brain, restoring cosmetic appearance, relieving headaches, and preventing brain herniation. Early repair may help alleviate symptoms of the "syndrome of the