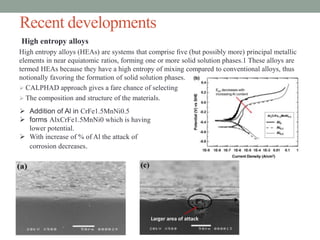
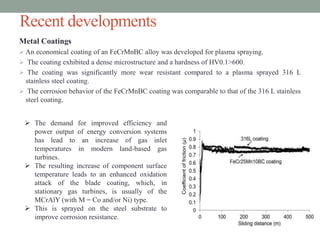
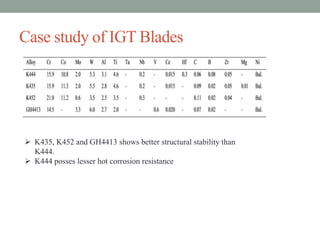
This document discusses corrosion resistant materials for extreme environments. It begins with an introduction that defines corrosion and extreme environments. It then reviews literature on the corrosion properties of various nickel alloys, high entropy alloys, and MCrAlY coatings. Next, it describes various corrosion resistant metals like stainless steels, nickel and aluminum alloys. It discusses recent developments in high entropy alloys and metal coatings. Finally, it provides two case studies on corrosion issues in nuclear power plants and gas turbine blades.



![Literature review
C.J.Liu et.al [1] used 4 different nickel alloys for IGT blades and studied their different
properties like hot corrosion, long term structural stability and other microstructural properties.
Pin Lu et.al [2] used integrated computational materials approach to find the chemical
composition and microstructure of the high entropy alloys to exhibit high corrosion properties.
Yao Qiu et.al [3] noticed that generally, in spite of complex compositions and in many cases
complicated microstructural heterogeneity, compositionally complex alloys are nominally
corrosion-resistant. This is discussed and aspects of the status and needs are presented.
H. Nickel et.al [4] studied that the demand for improved efficiency and power output of energy
conversion systems has lead to an increase of gas inlet temperatures in modern land-based gas
turbines. Isothermal and cyclic oxidation tests were carried out in the temperature range 950°C-
1100°C on MCrAlY coatings. The effect of systematic variation of titanium and silicon
contents on oxidation and micro structural stability was studied by characterization of the
coating and the corrosion products using light and electron optical microscopy and by
secondary neutrals mass spectrometry (SNMS).
Kirsten Bobzin [5] et.al described the wear and corrosion resistant properties of FeCrMnBC
coatings and its economical advantage and superior properties over stainless steel 316L.
Vivekanand Kain[6] discussed the type of corrosions and types of materials which resists the
corrosion phenomena , its advantages and disadvantages.](https://image.slidesharecdn.com/corrosionresistantmaterials-190323182011/85/Corrosion-resistant-materials-4-320.jpg)