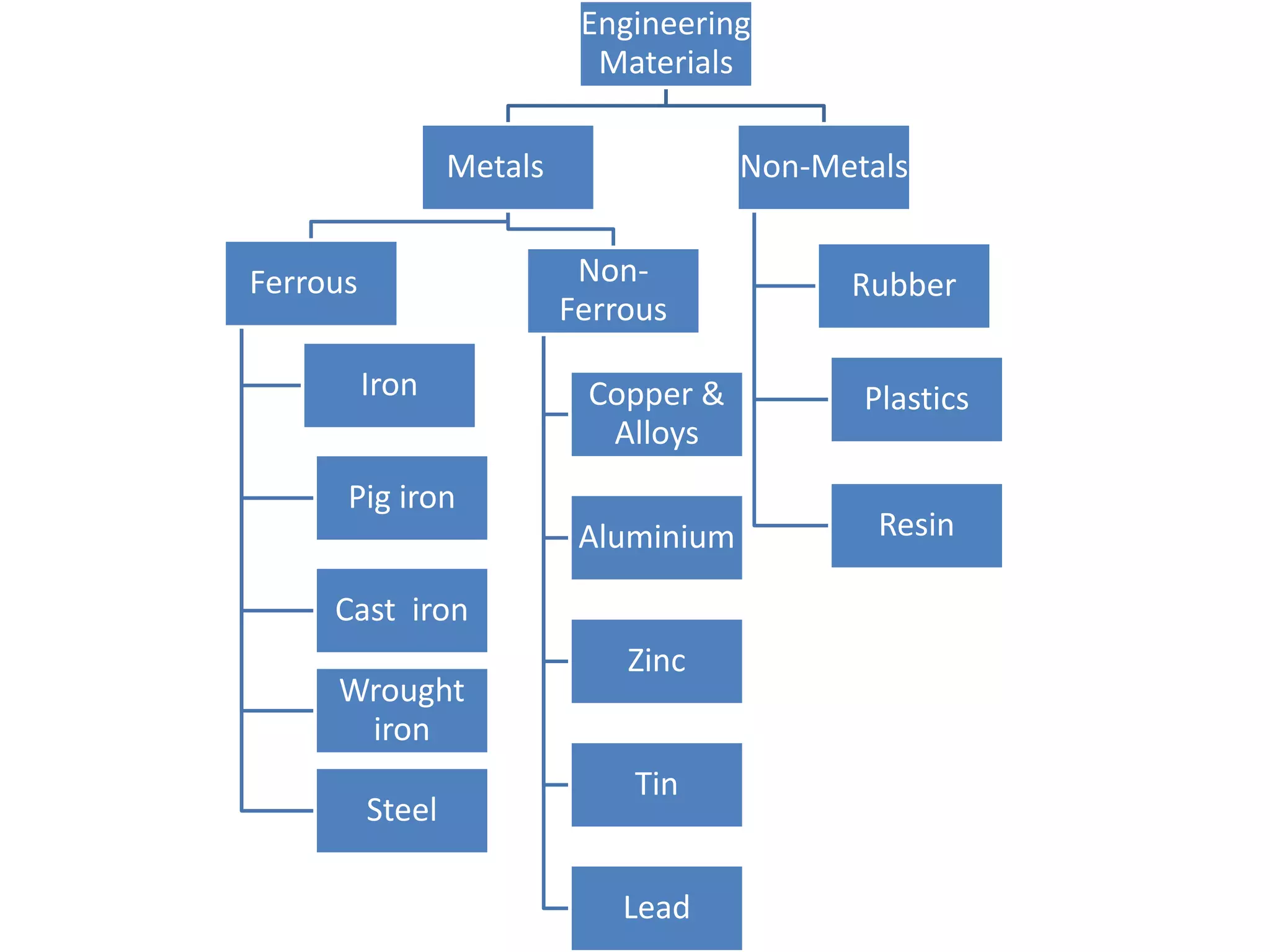
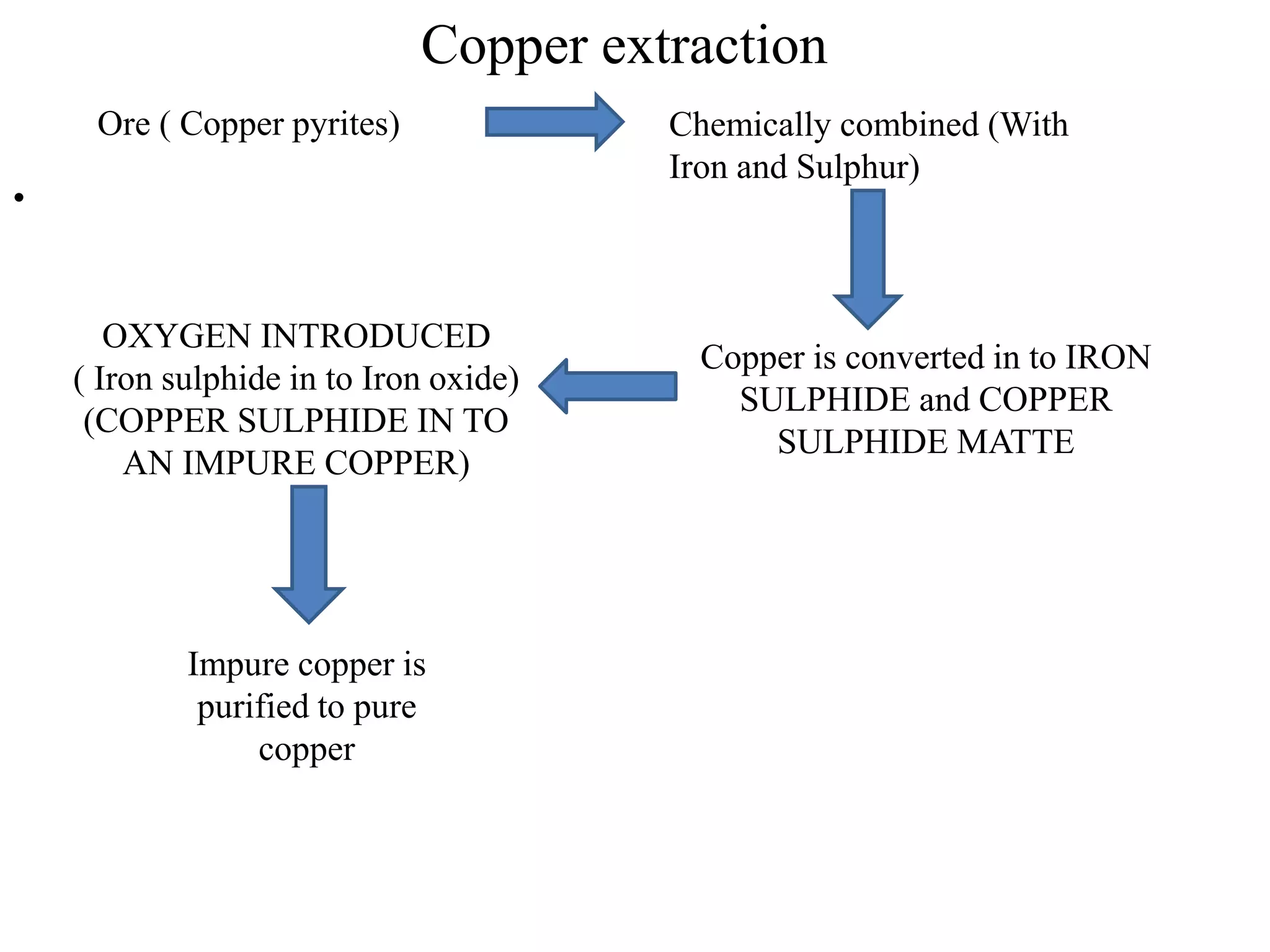
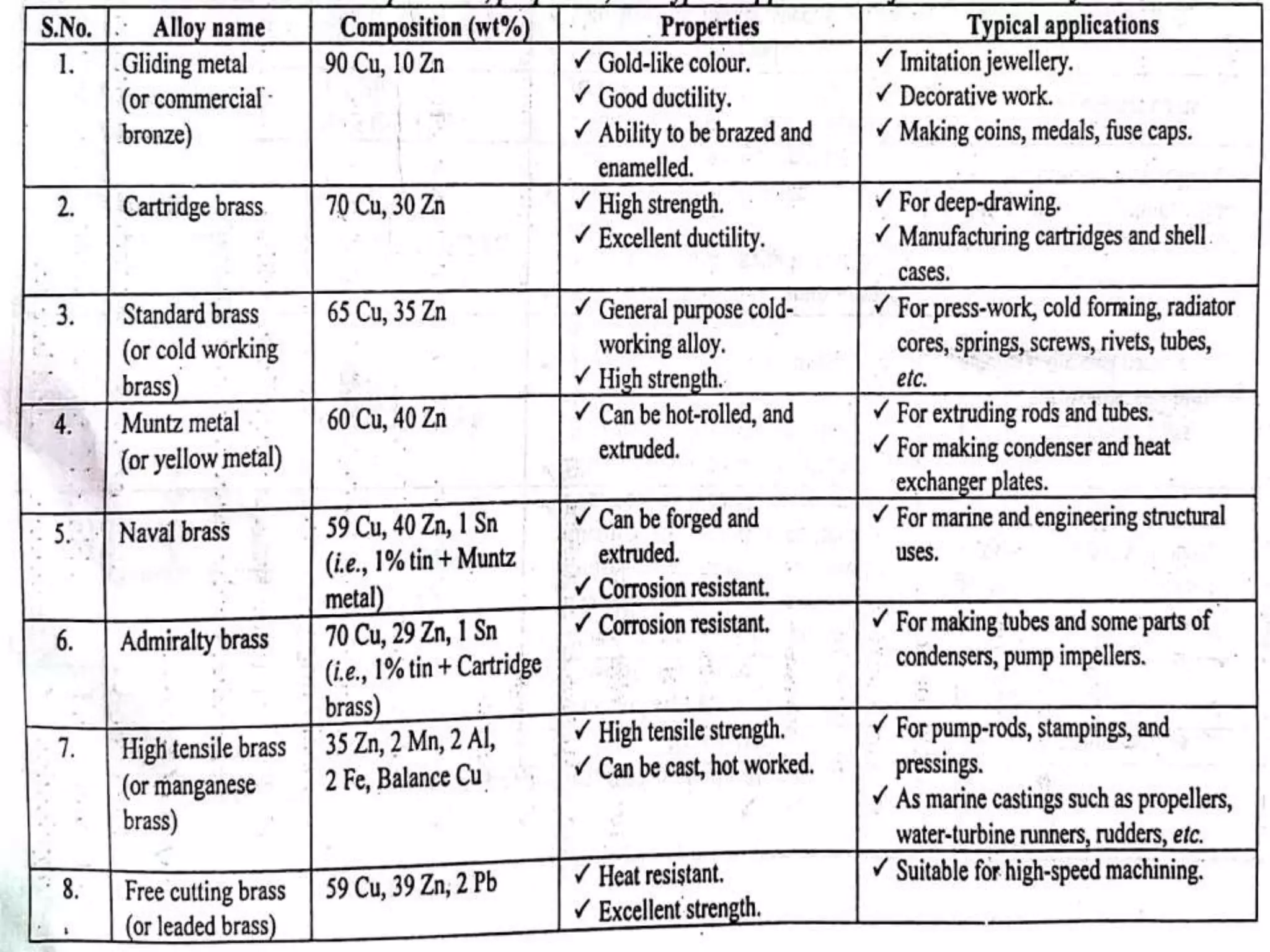
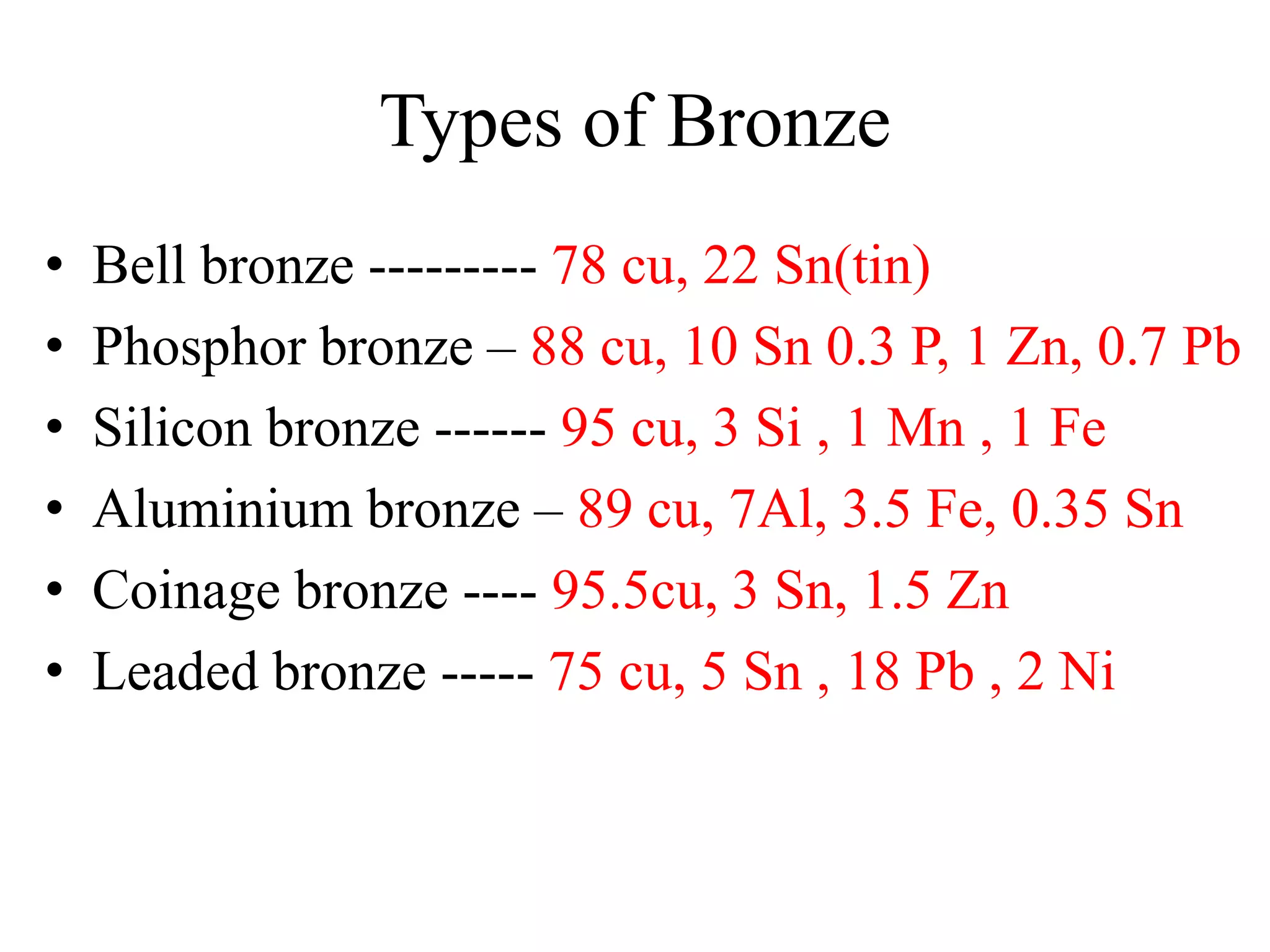
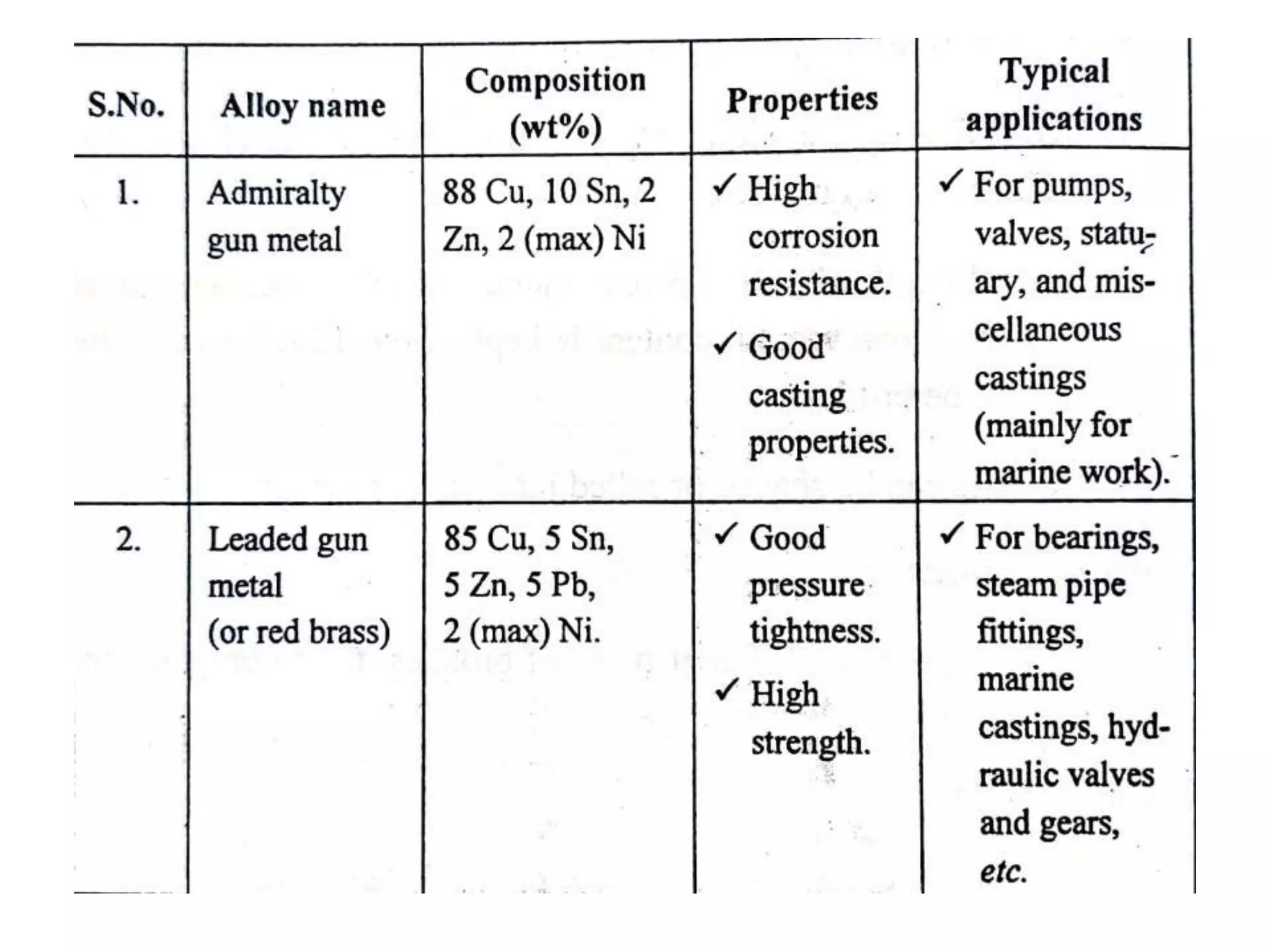
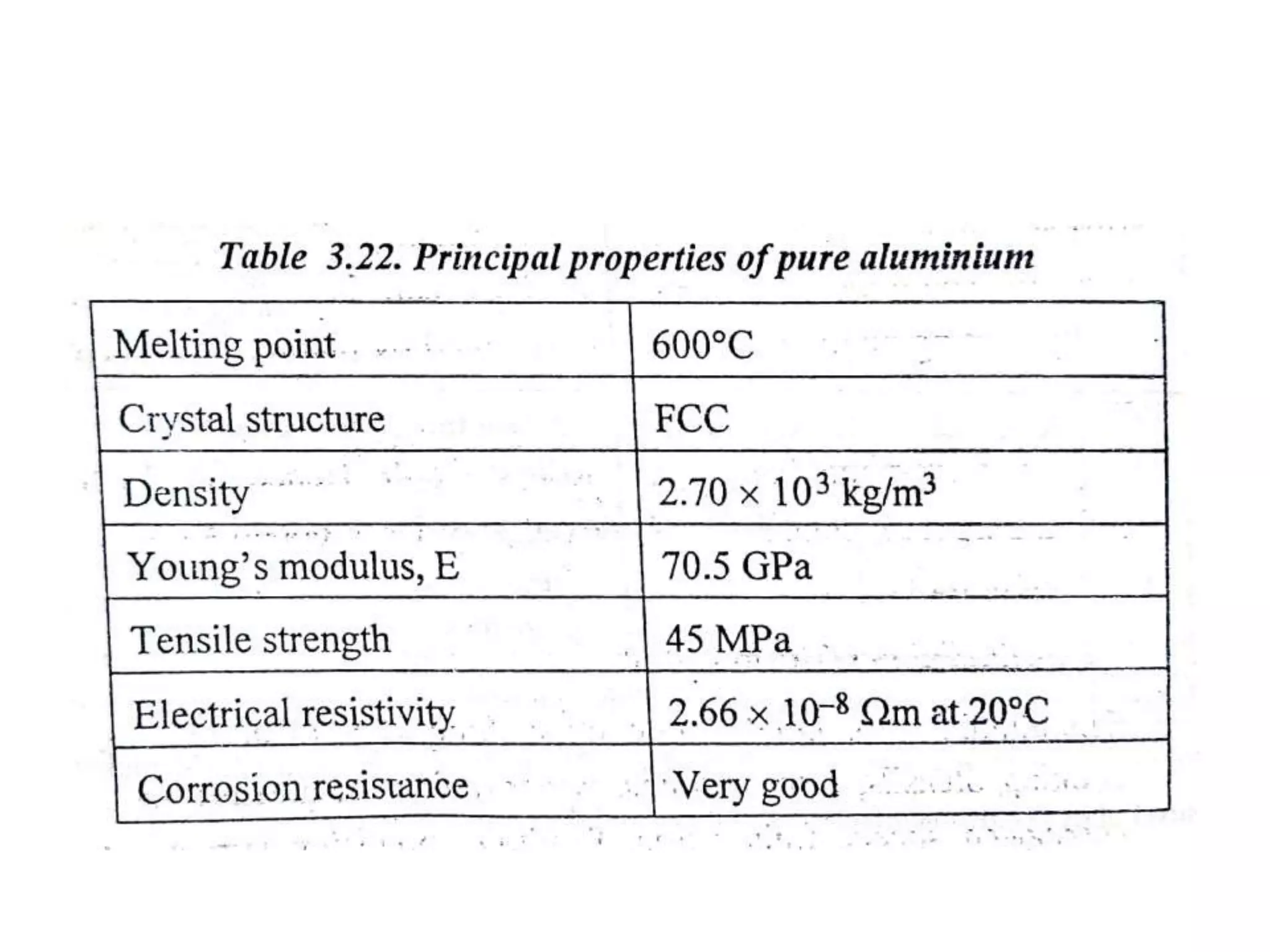
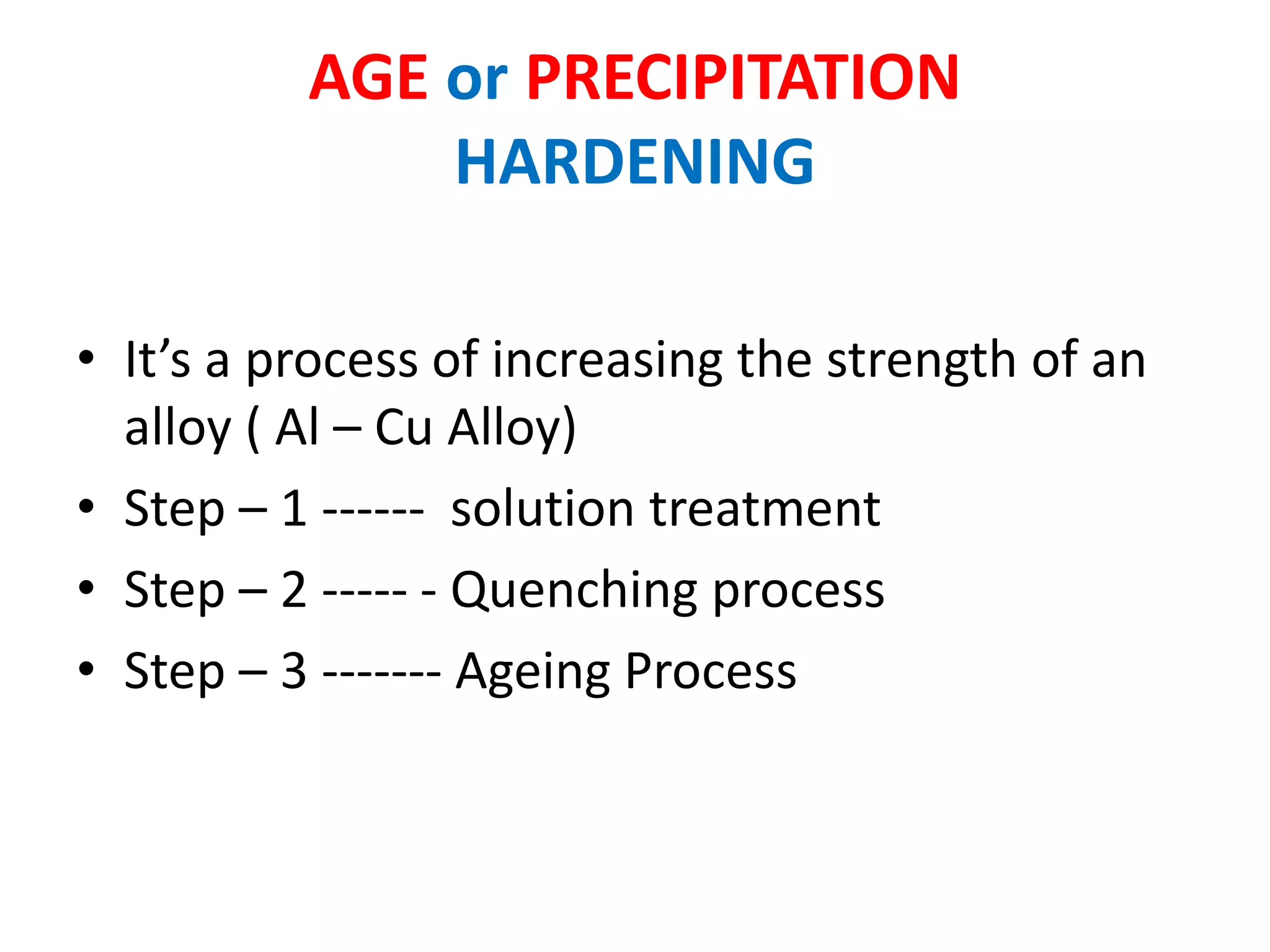
This document provides information on non-ferrous metals and their properties and applications. It discusses copper, its extraction process, properties, and common alloys like brass, bronze, gun metal and cupronickel. It also covers aluminium, describing its extraction, properties, common alloys and uses. Bearing materials are discussed last, outlining characteristics of bearing materials and common types used like white metals, copper alloys, aluminium alloys, plastics and ceramics.