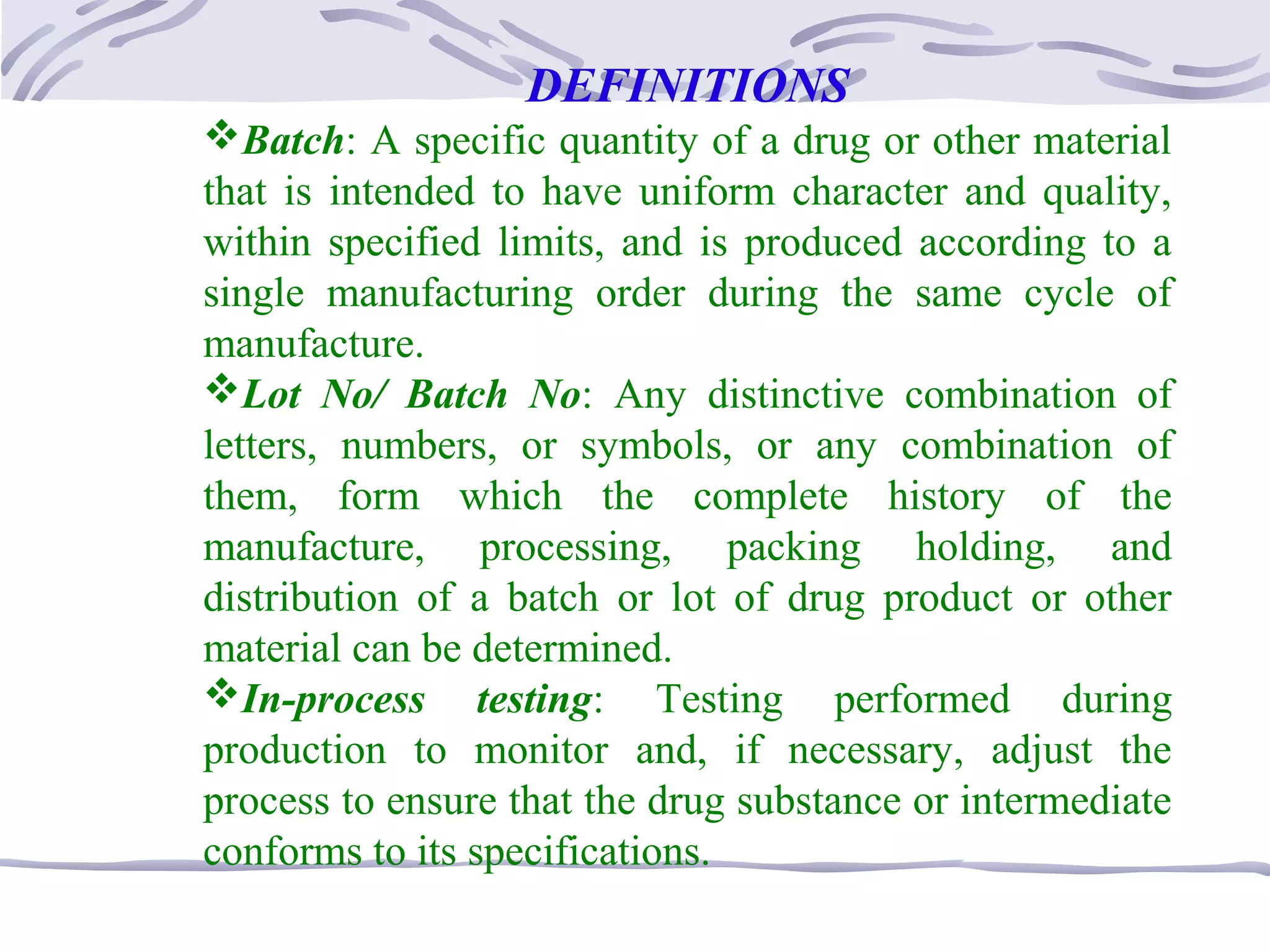
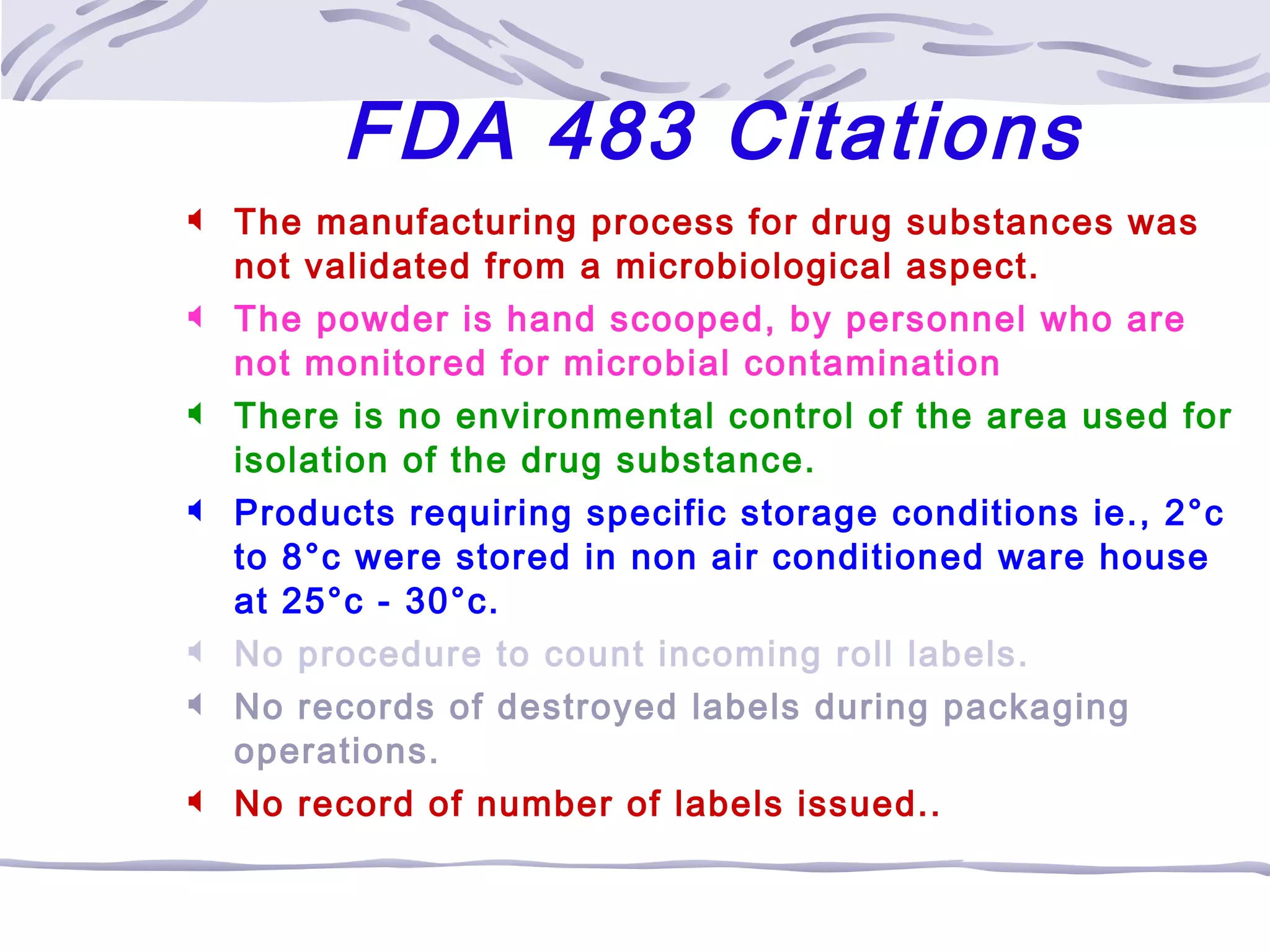
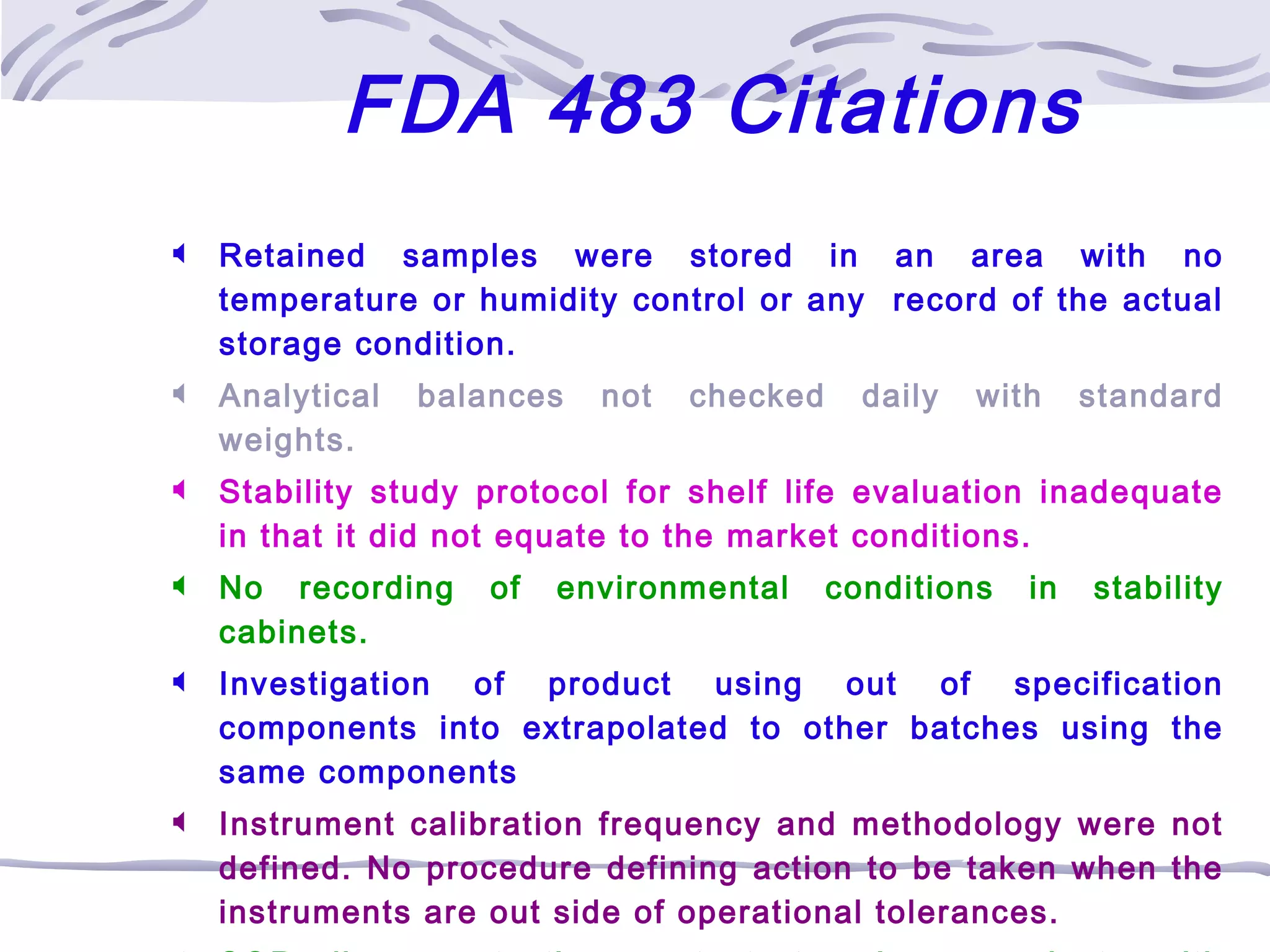
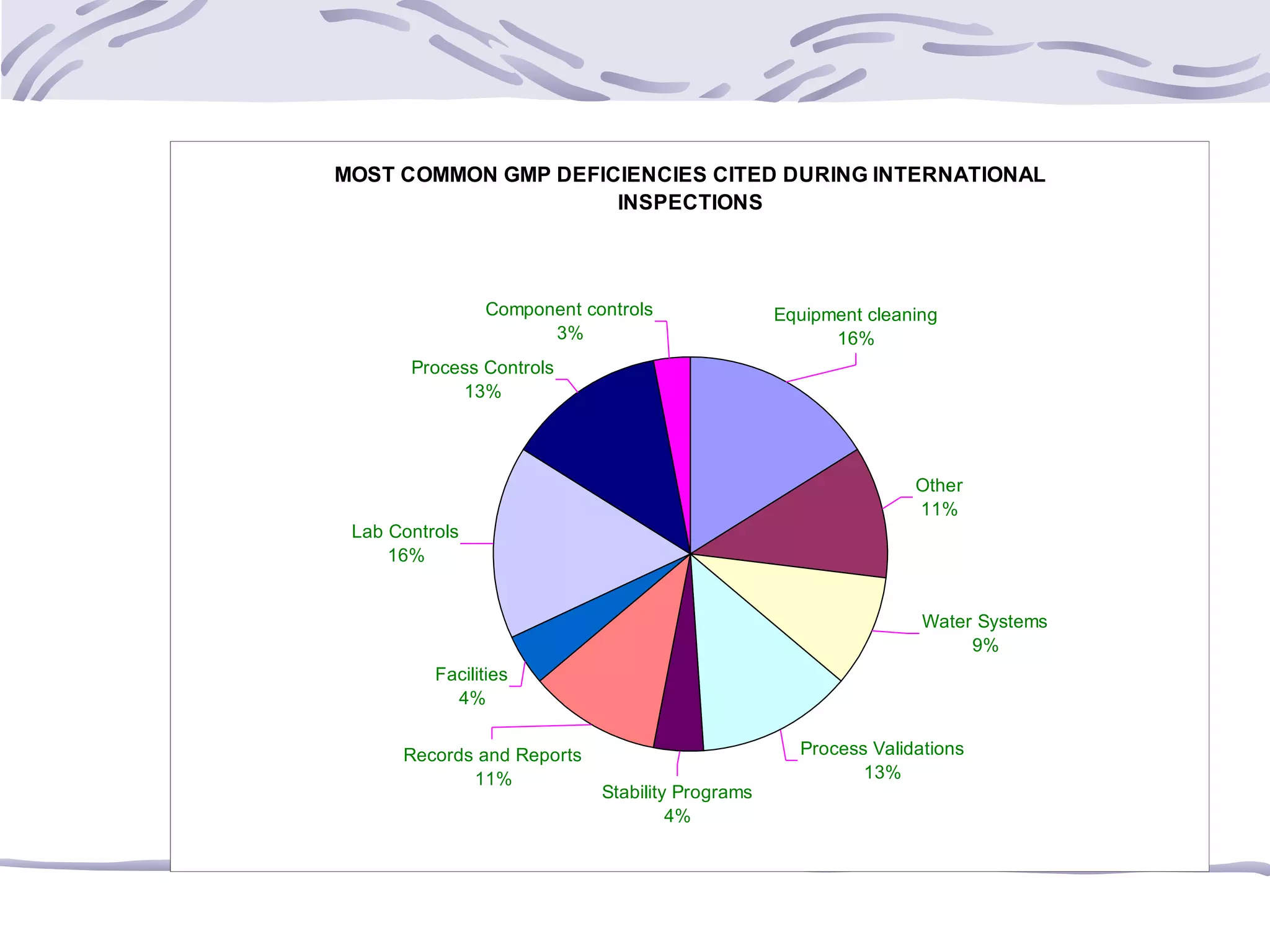
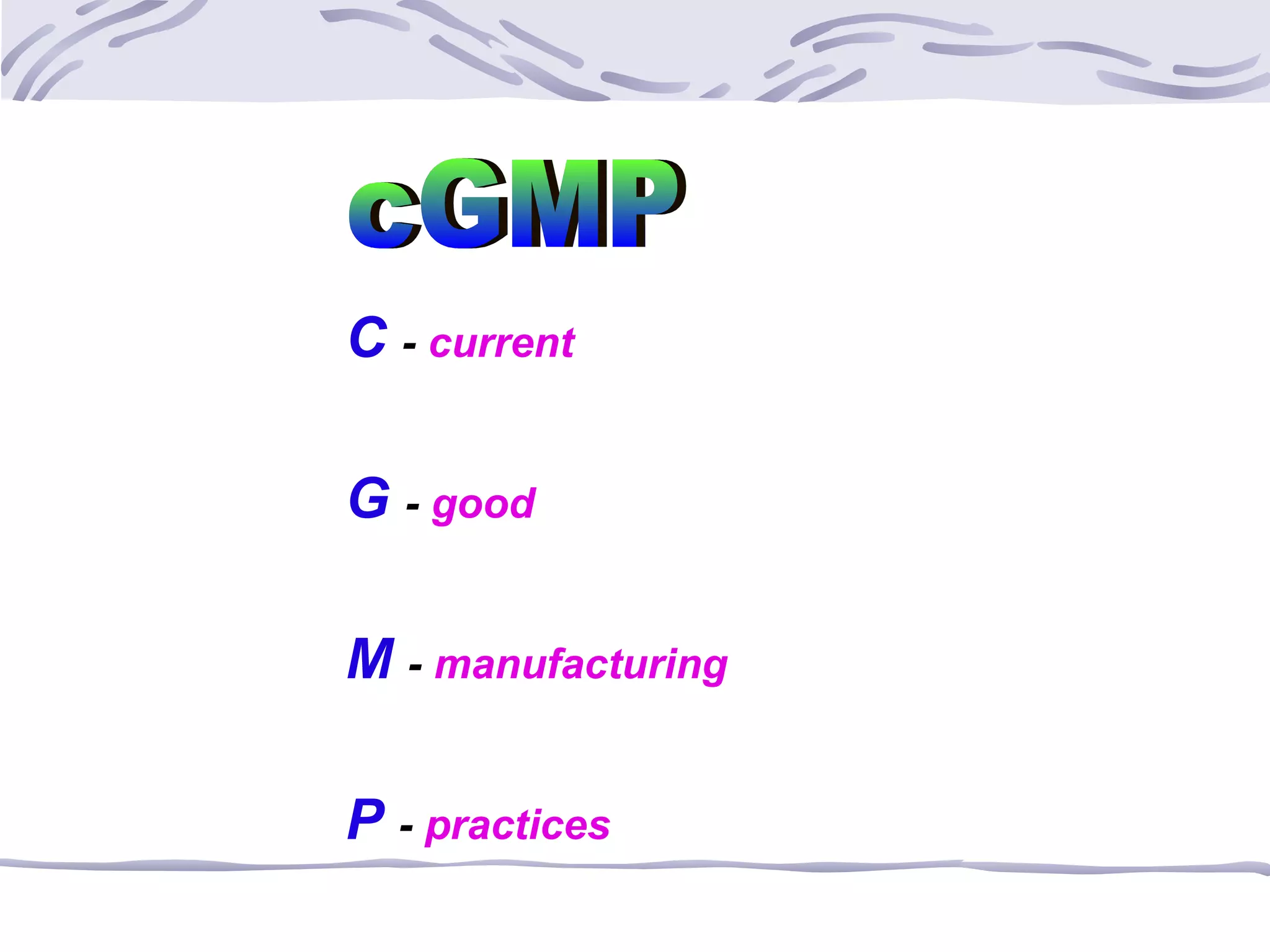
The document provides an overview of the training program on 21 CFR Parts 210 and 211, which covers current Good Manufacturing Practices (cGMP) for drug manufacturing and quality assurance. It details regulations, definitions, and requirements related to personnel qualifications, production controls, packaging, label management, laboratory controls, and record-keeping necessary to ensure drug products meet safety and quality standards. Additionally, it highlights common deficiencies observed in international GMP inspections and emphasizes the significance of compliance with these regulations to avoid regulatory actions.






![210.2 Applicability of CGMP
Regulations.
01. The regulation in this part may pertain to a
biological product for human use shall be consider as
supplement.
02. If the person engages in only some operations
subject to the regulation in this part that person need
only comply with these regulations applicable to the
operations in which he or she is engaged.
[ 600 parts exist but has to verify how many applicable ]](https://image.slidesharecdn.com/21cfr-150923031752-lva1-app6892/75/21-CFR-FOOD-AND-DRUG-ADMINISTRATION-DEPARTMENT-OF-HEALTH-AND-HUMAN-SERVICES-UNITED-STATES-OF-AMERICA-9-2048.jpg)