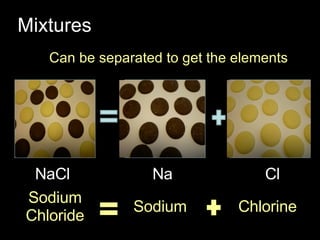
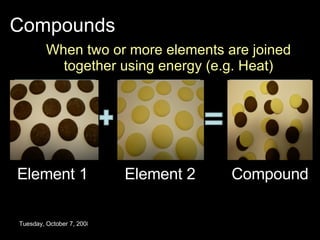
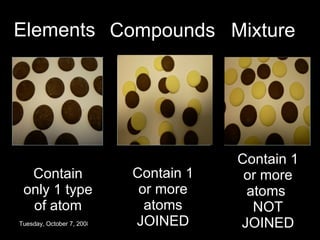
The document discusses elements, compounds, and mixtures. It explains that elements contain only one type of atom, while compounds contain two or more different elements that are chemically bonded together. Mixtures contain elements or compounds mixed together but not chemically bonded. Seawater is described as a mixture of the compounds H2O and NaCl. The learning outcomes are to explain the differences between elements, compounds, and mixtures and how formulae indicate the number and type of atoms in compounds.