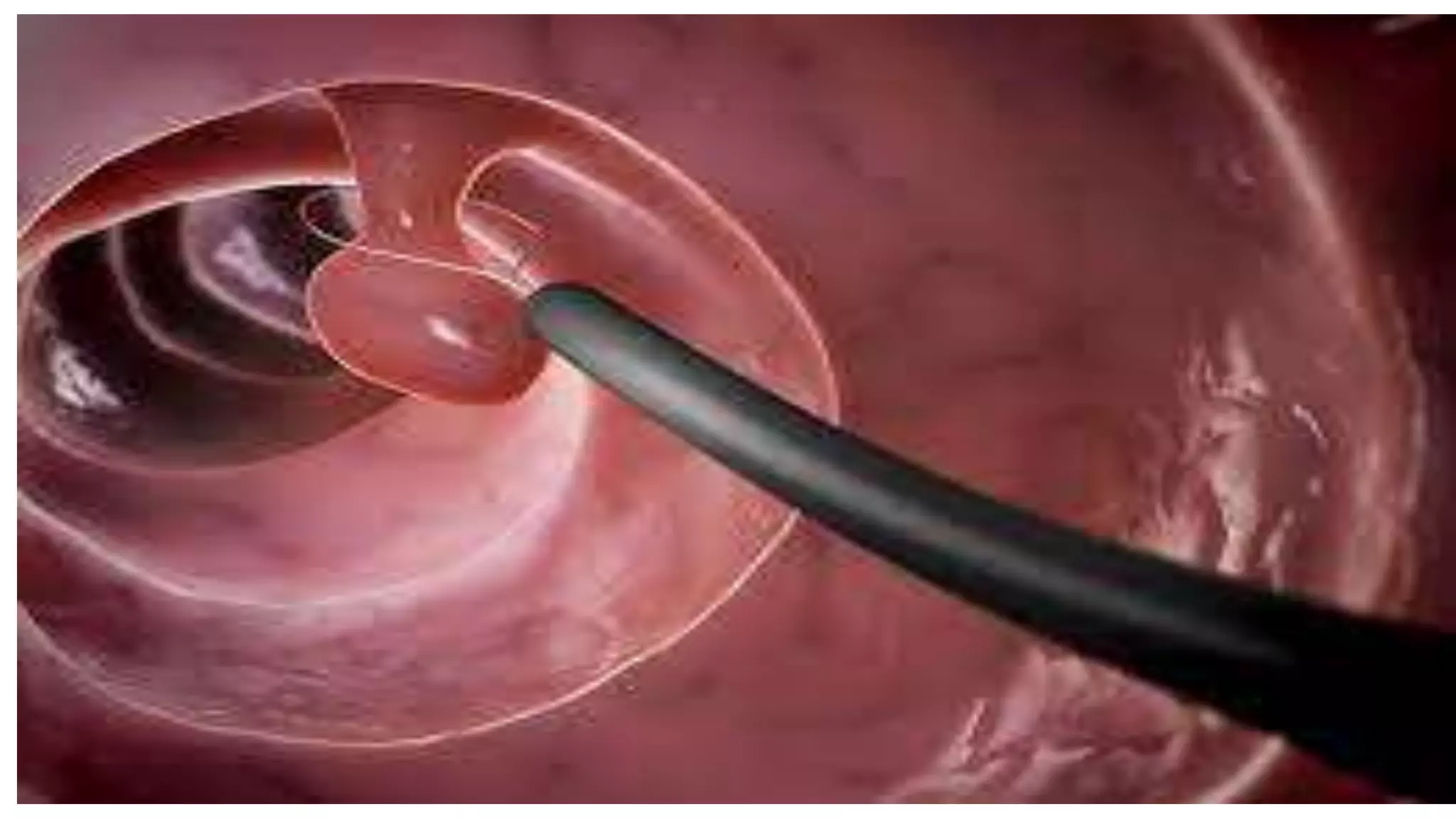
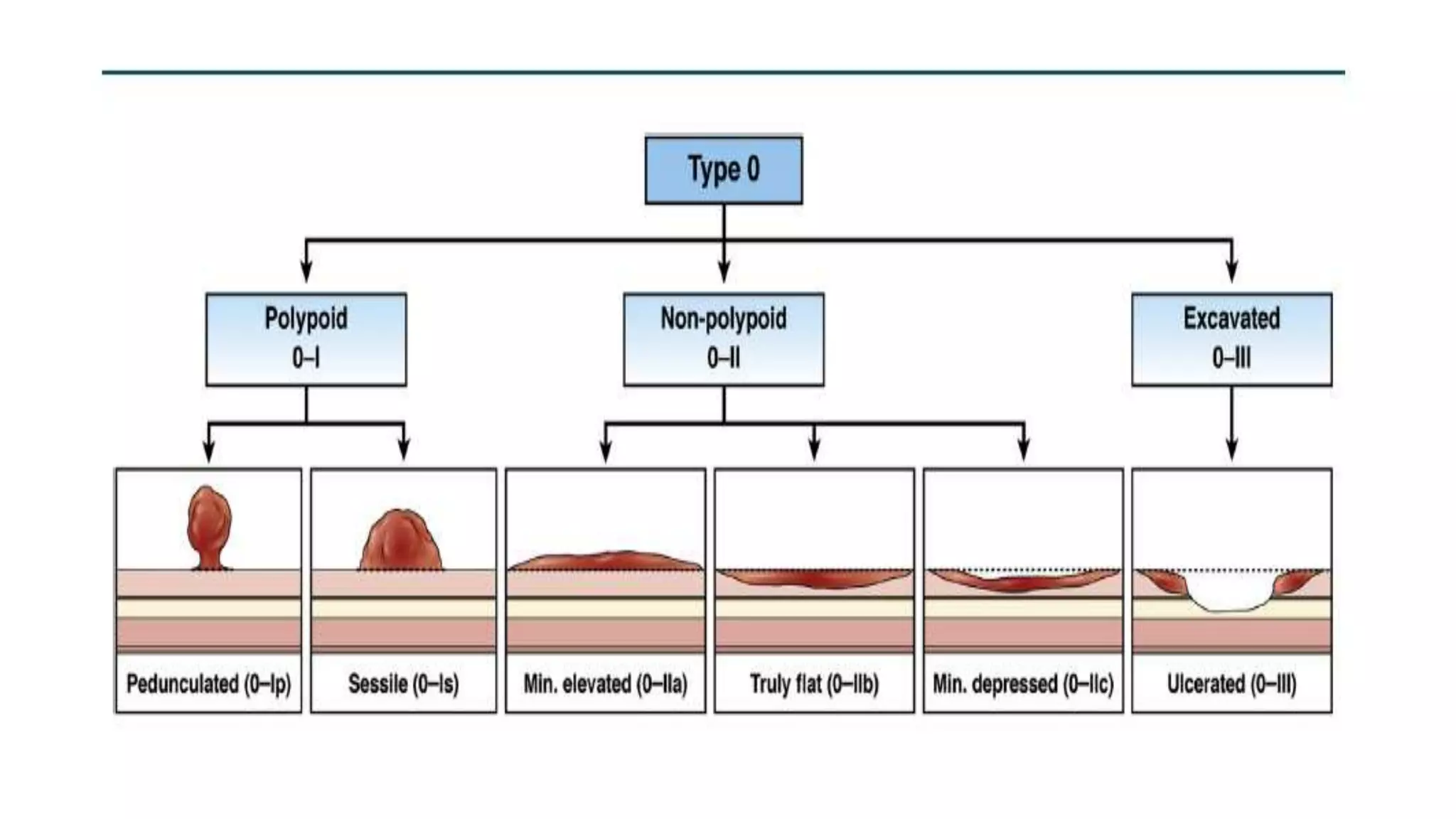
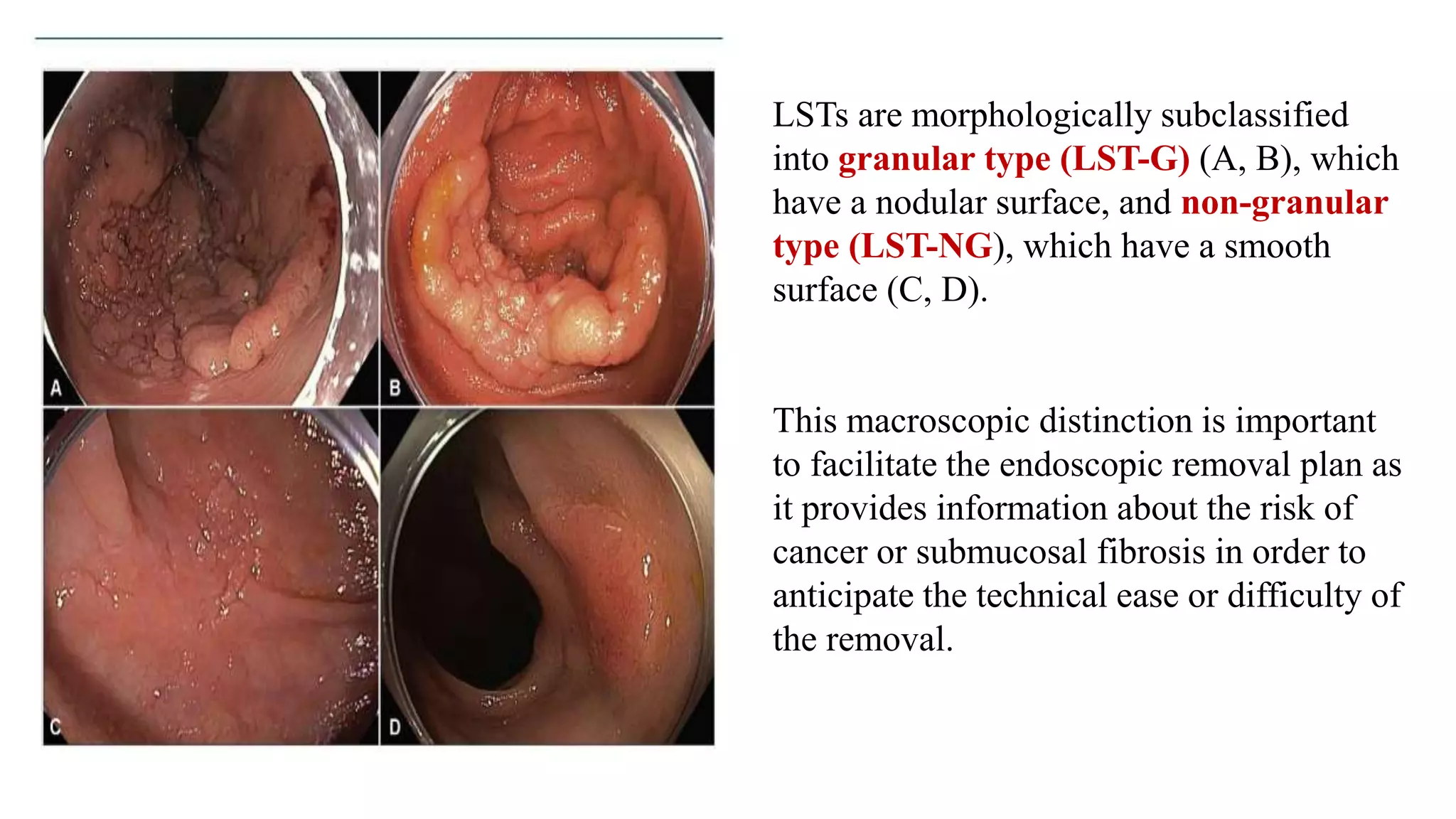
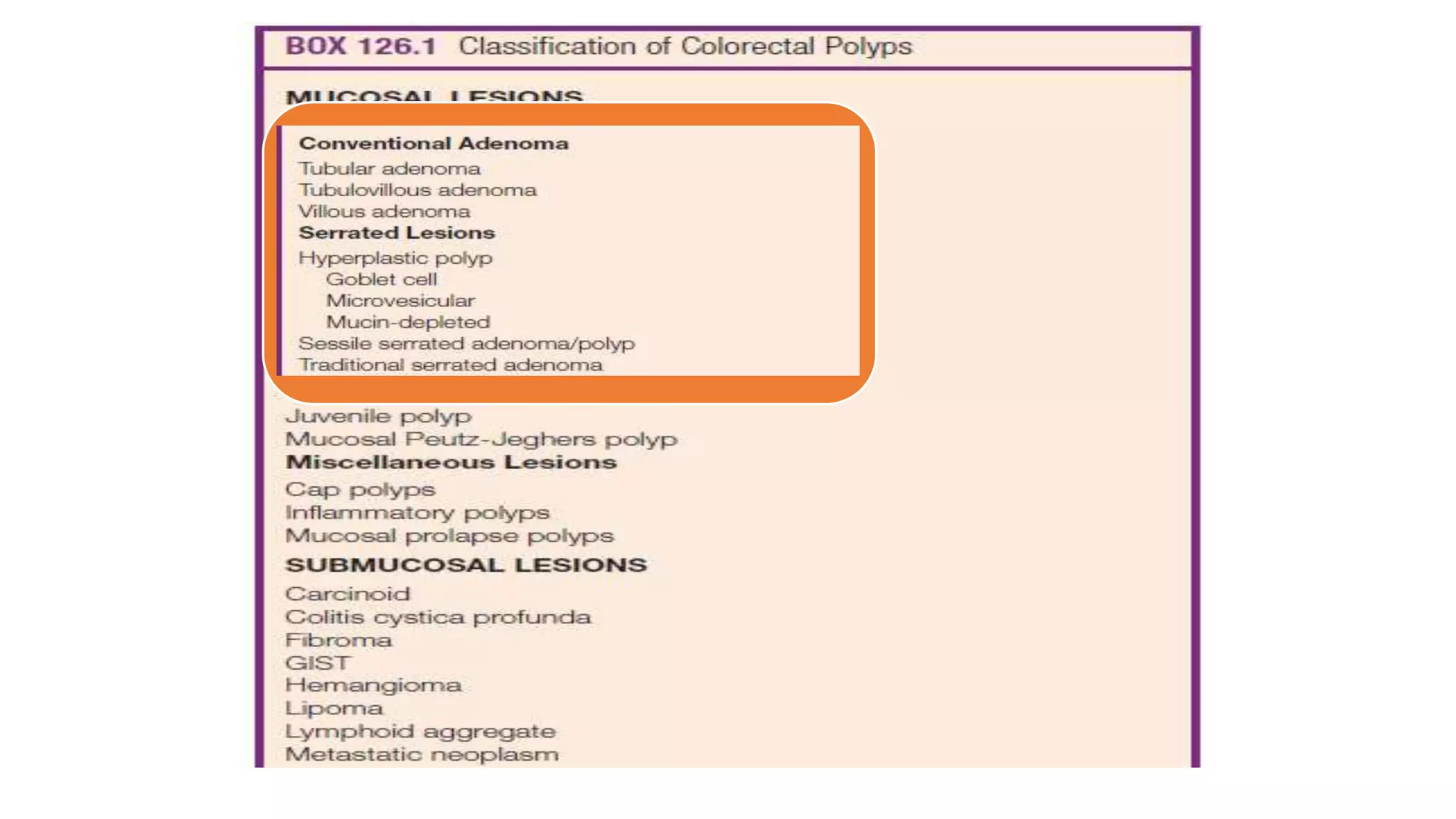
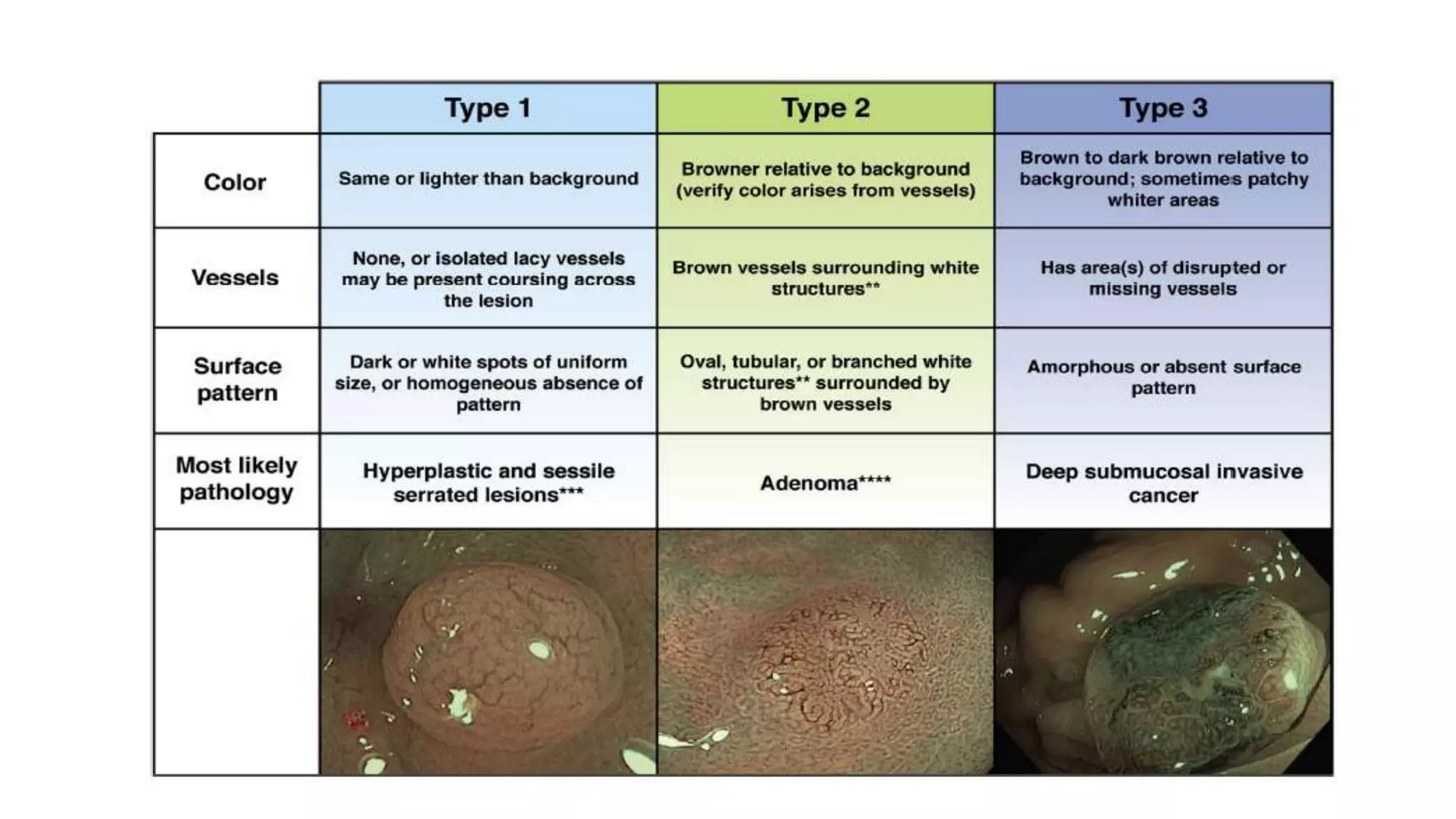
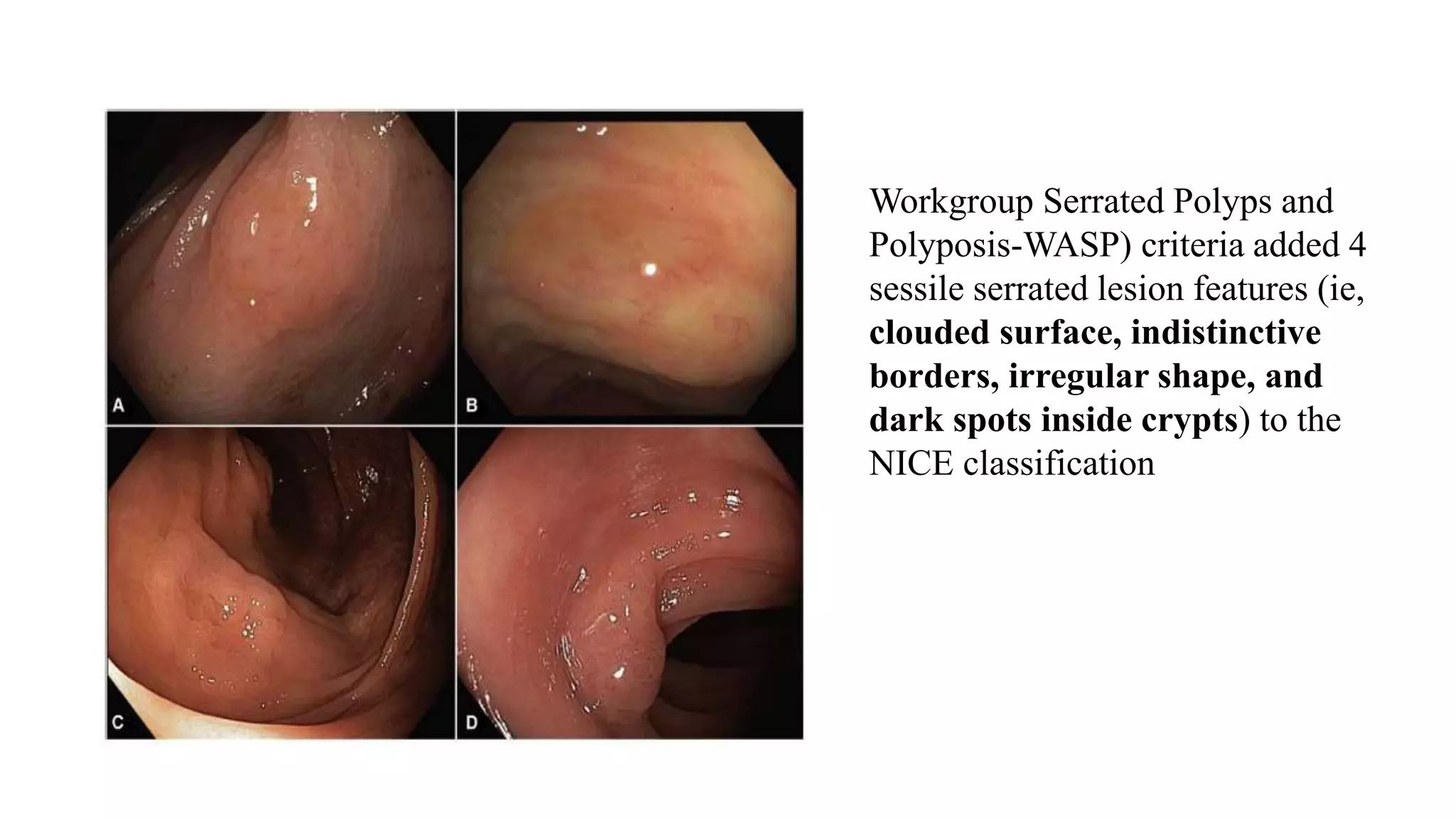
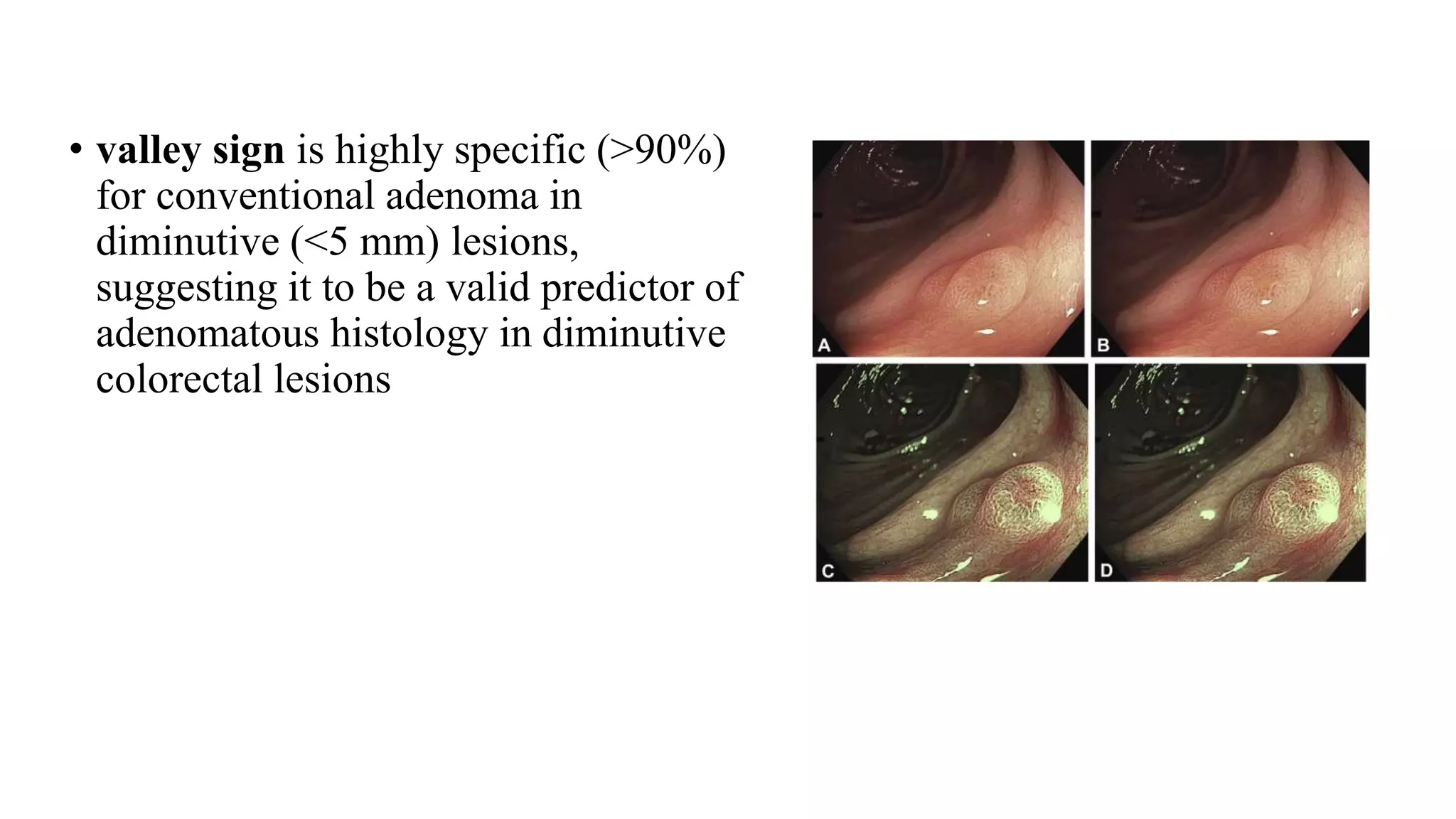
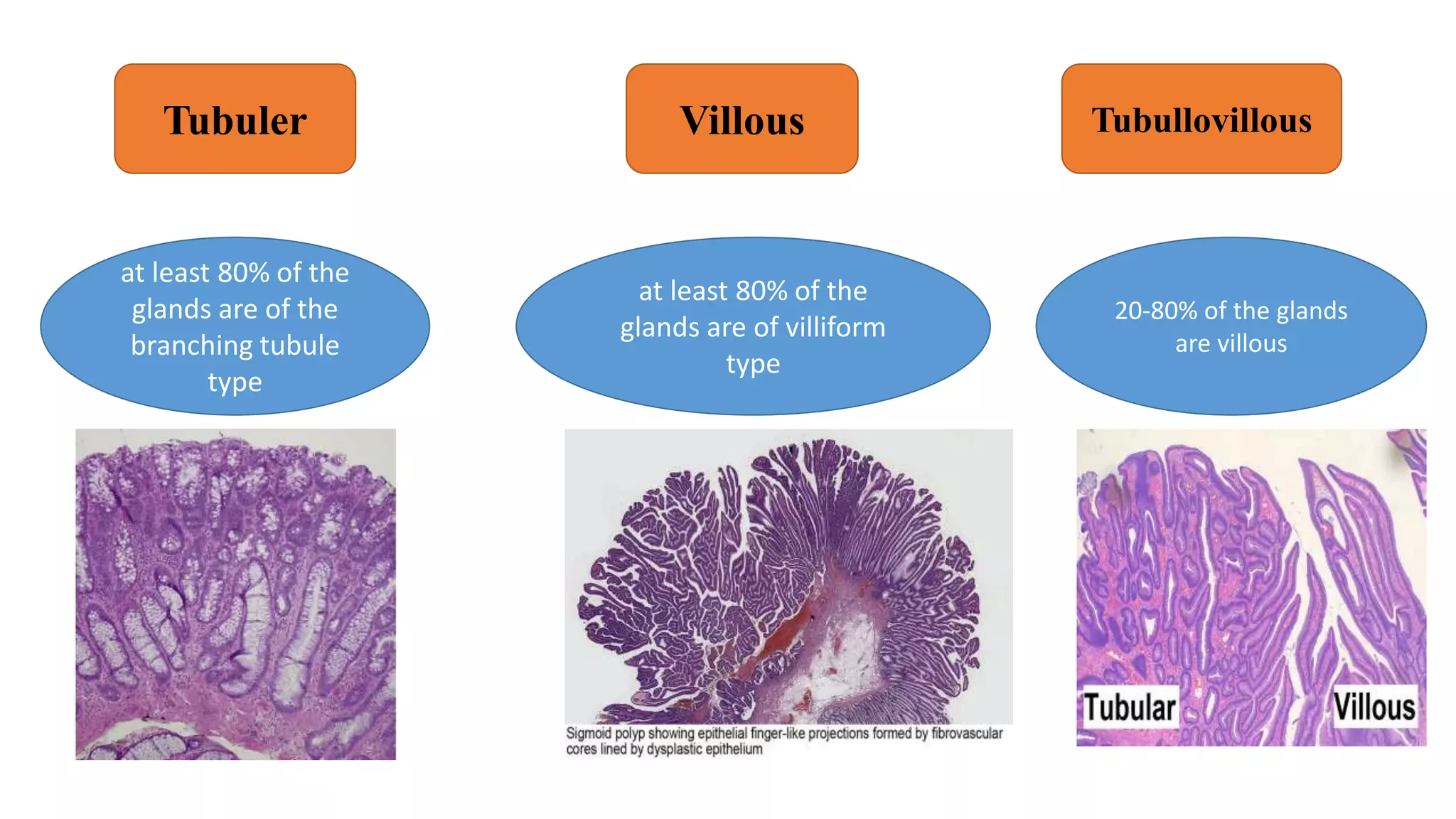
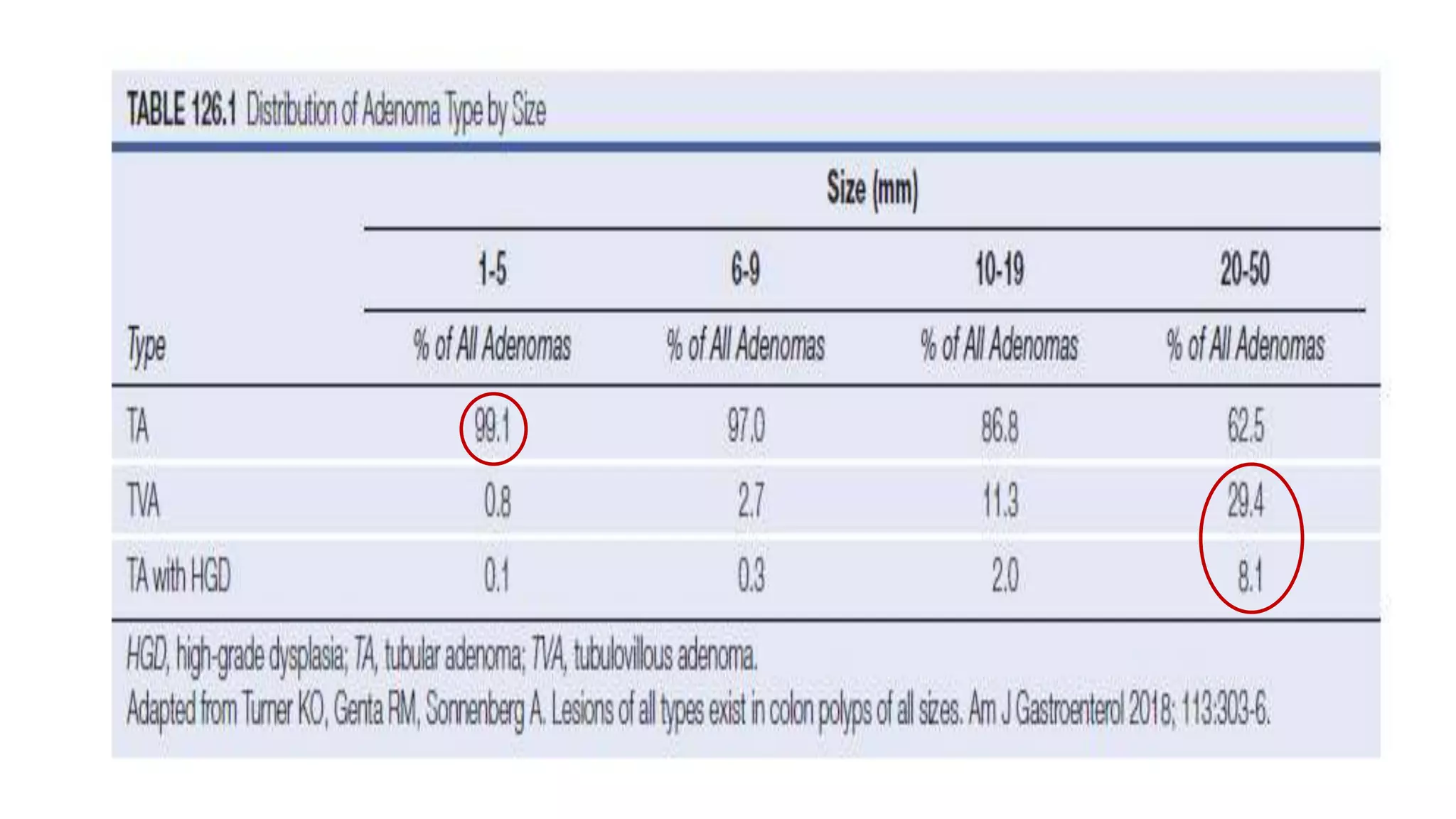
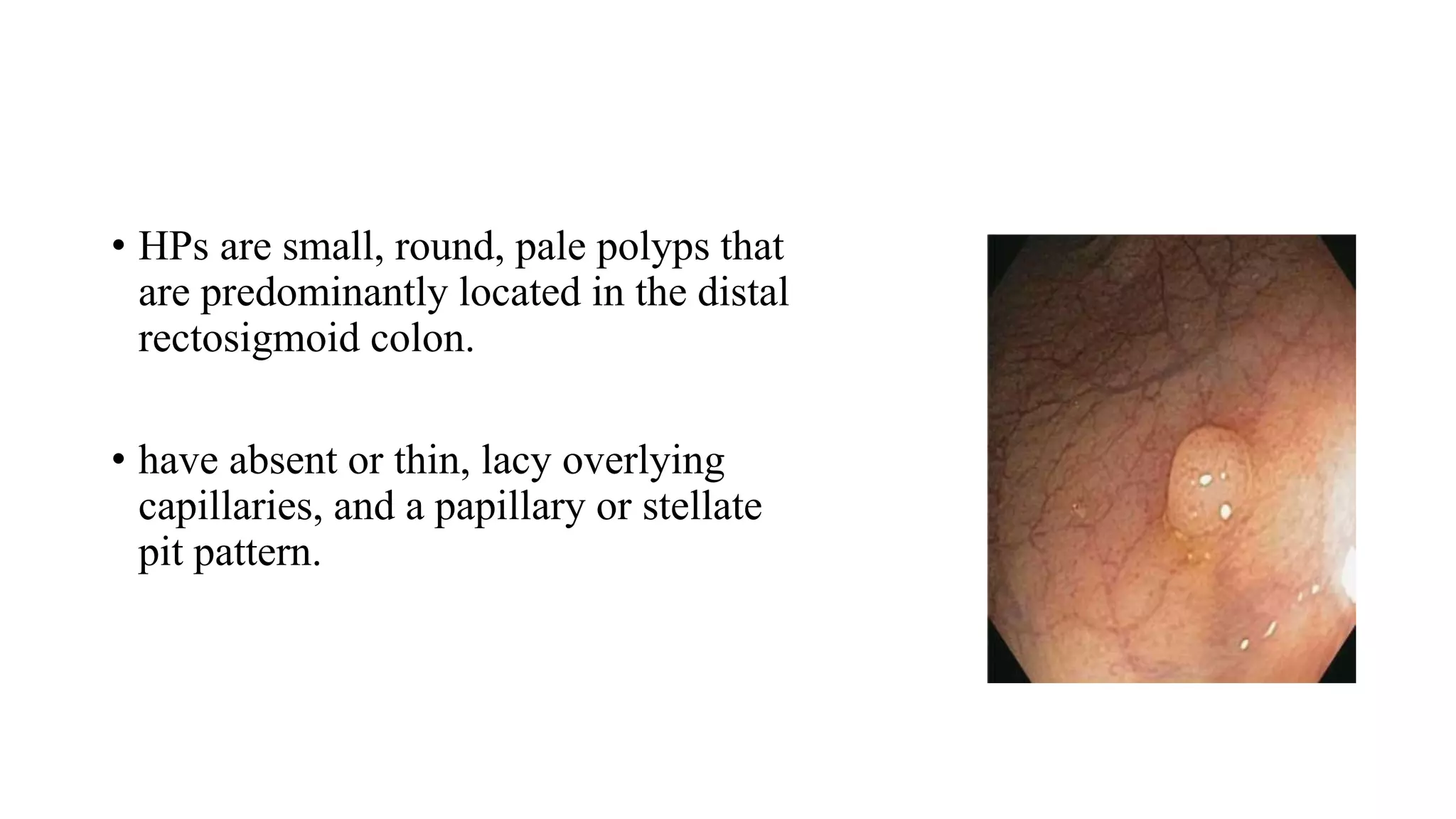
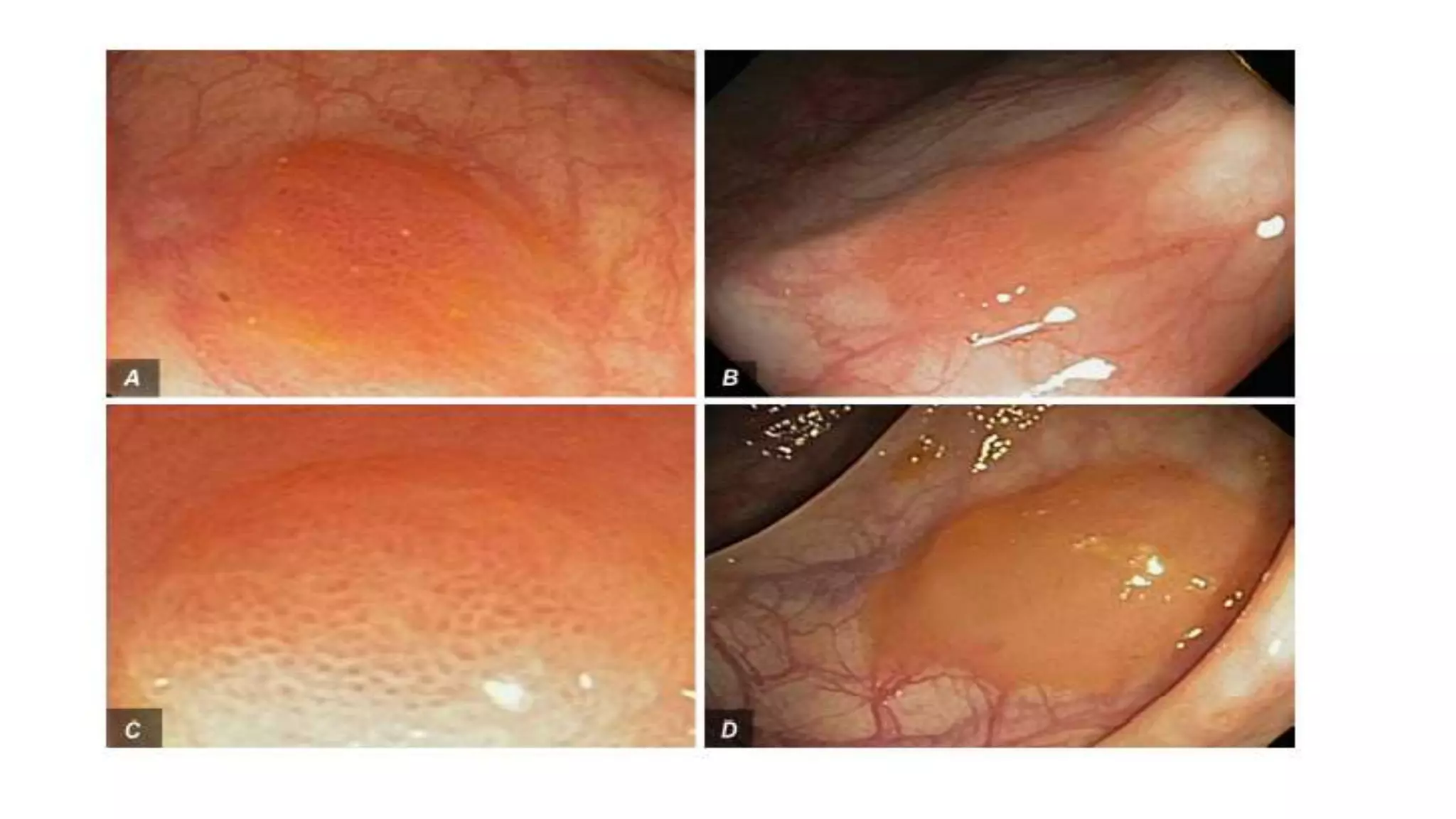
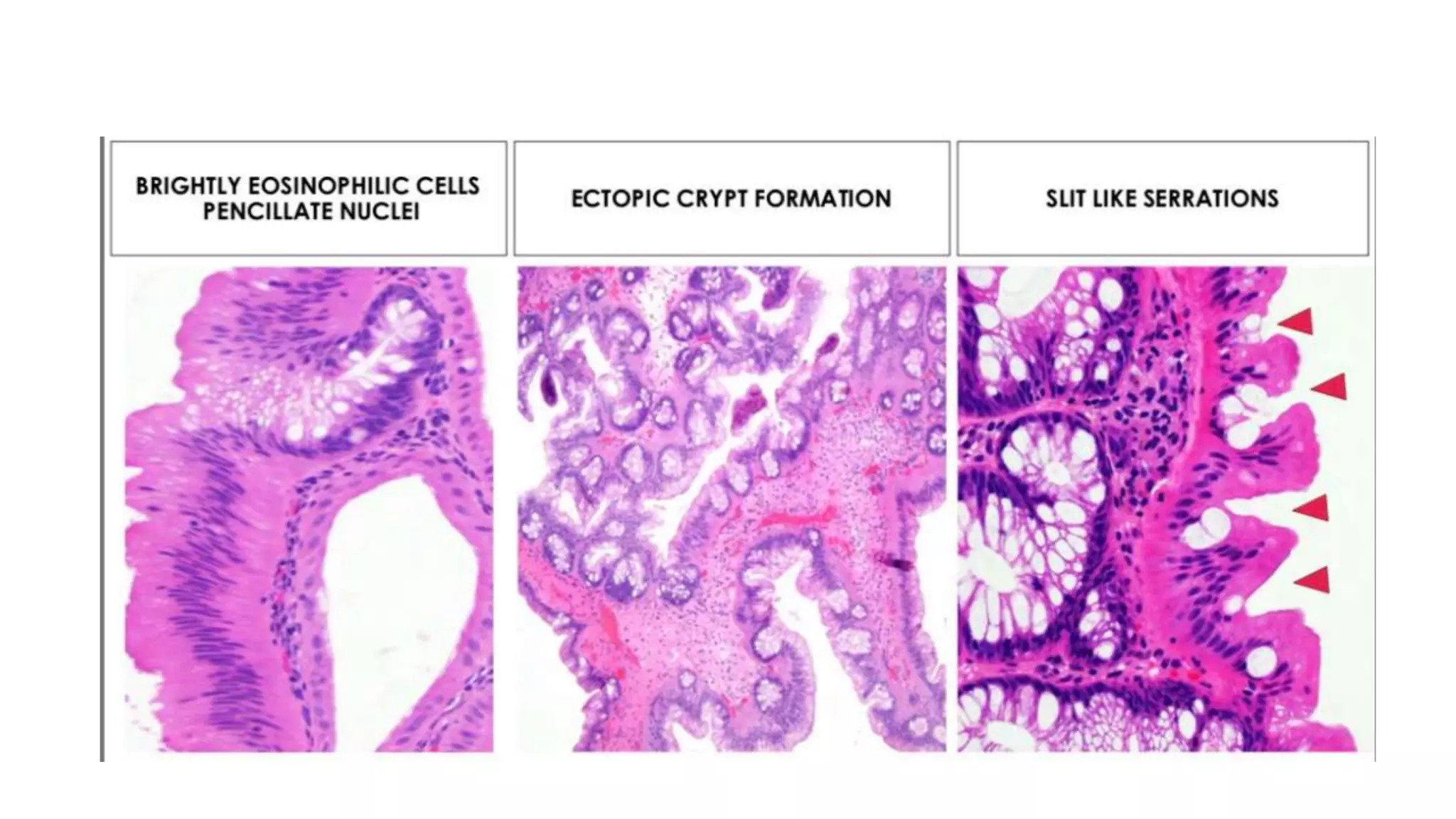
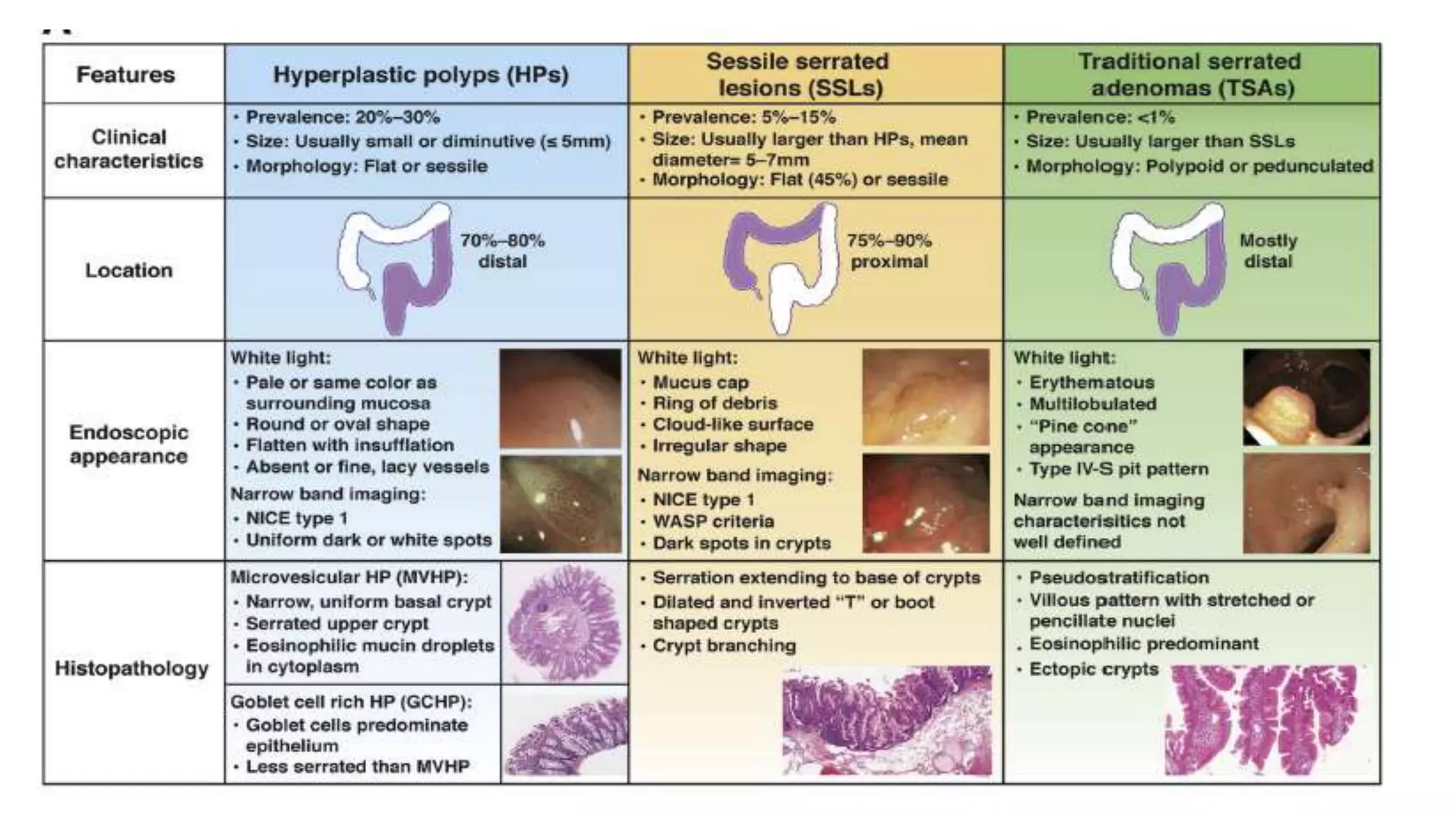
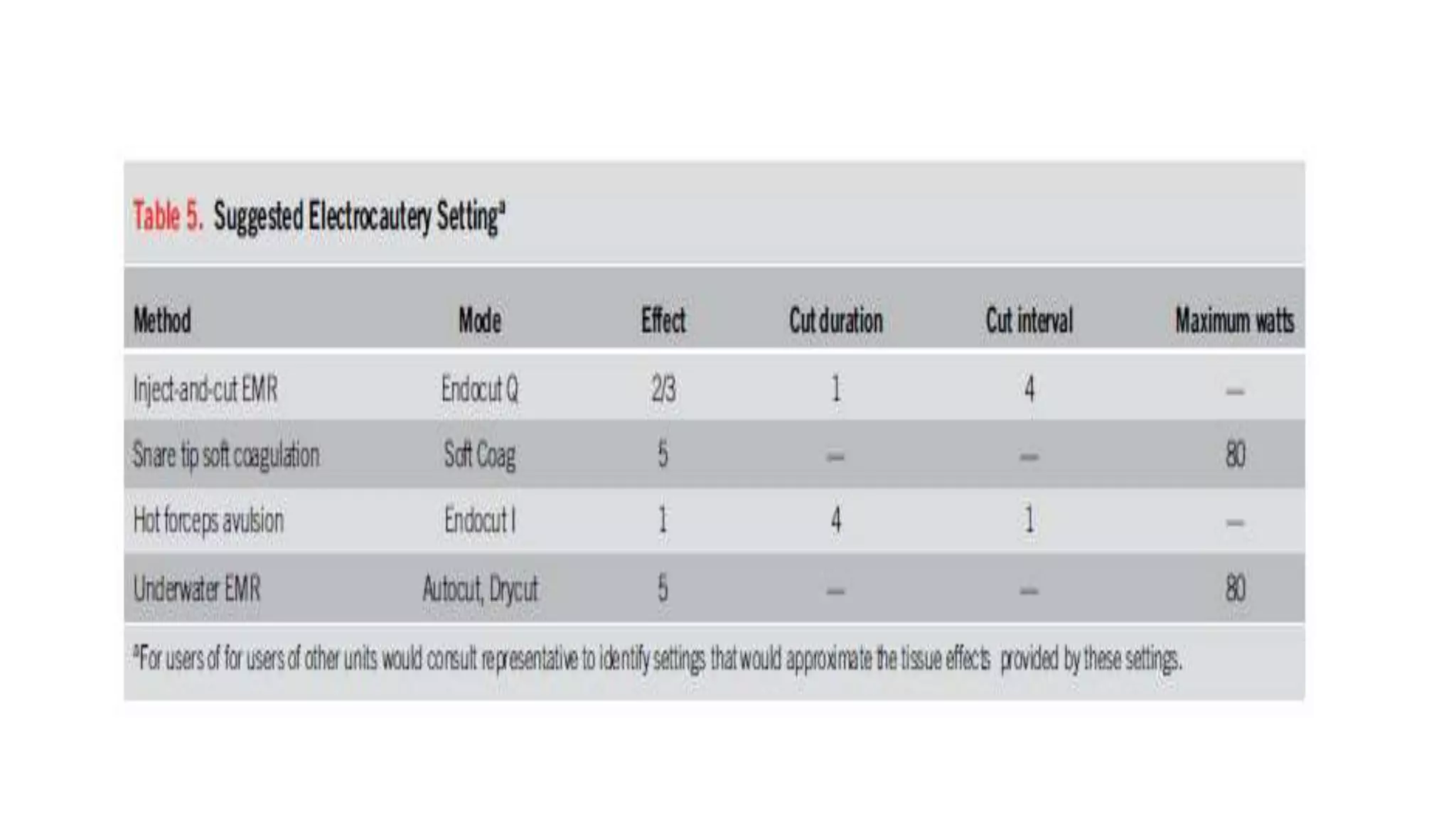
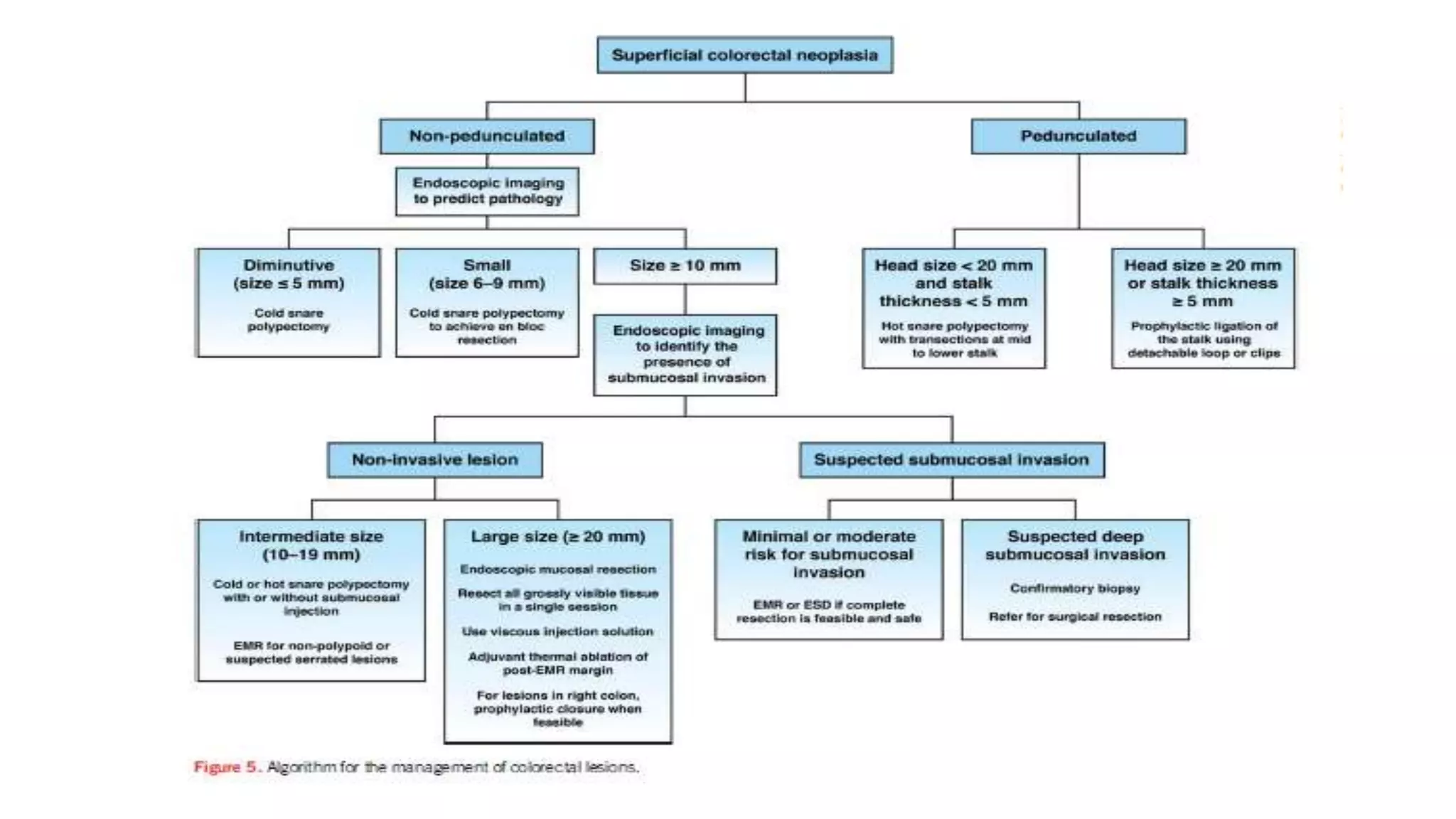
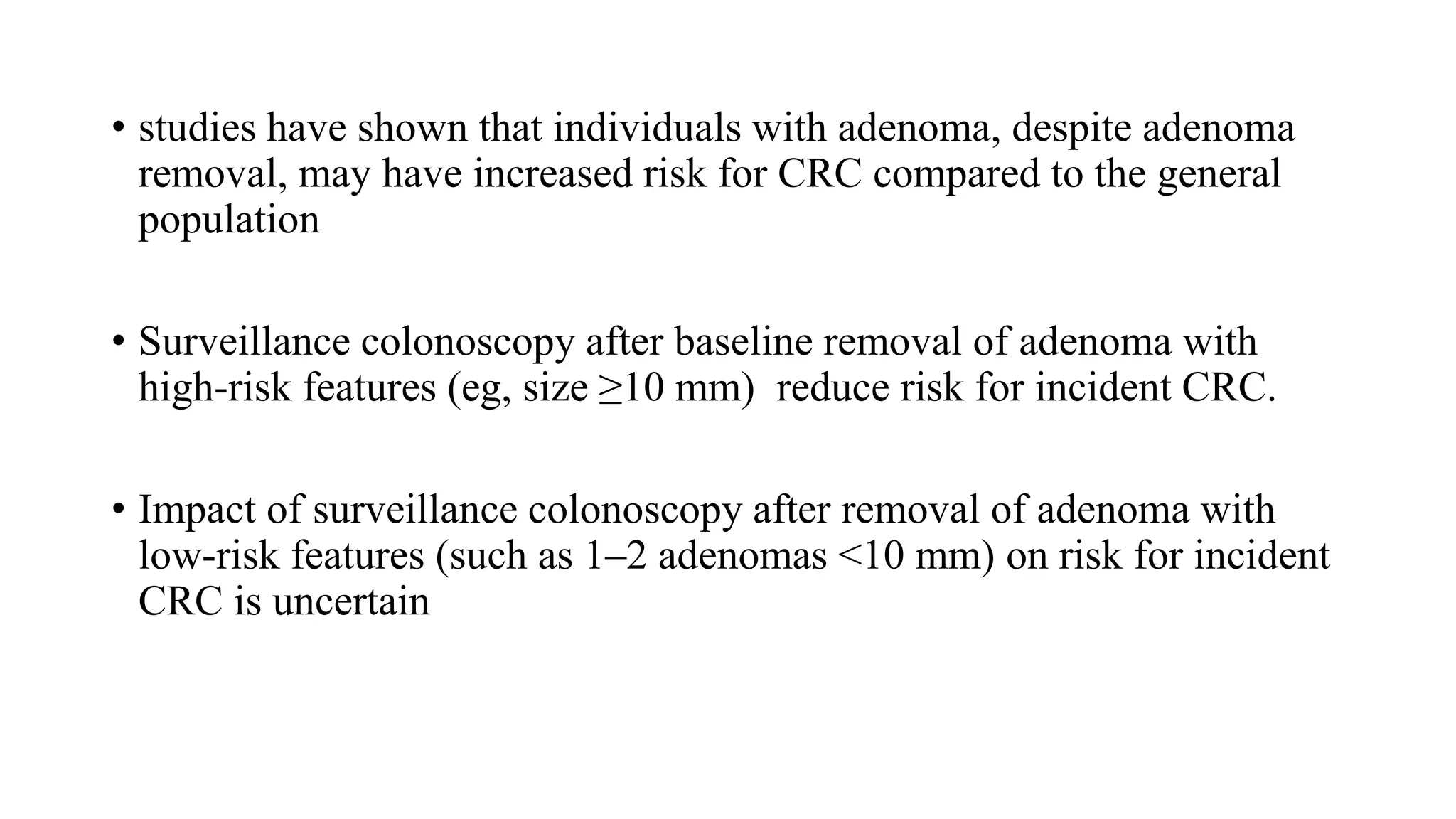
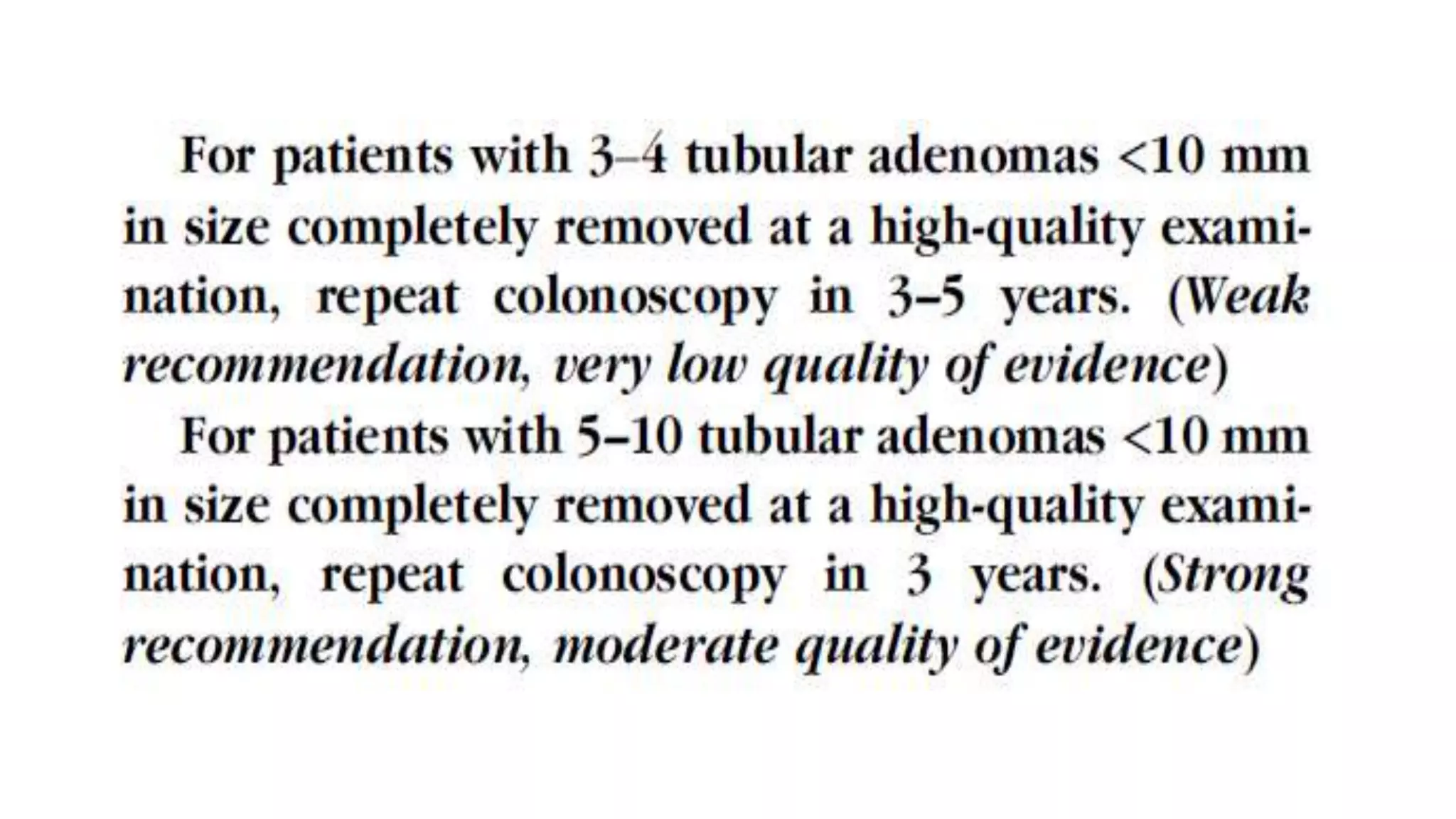
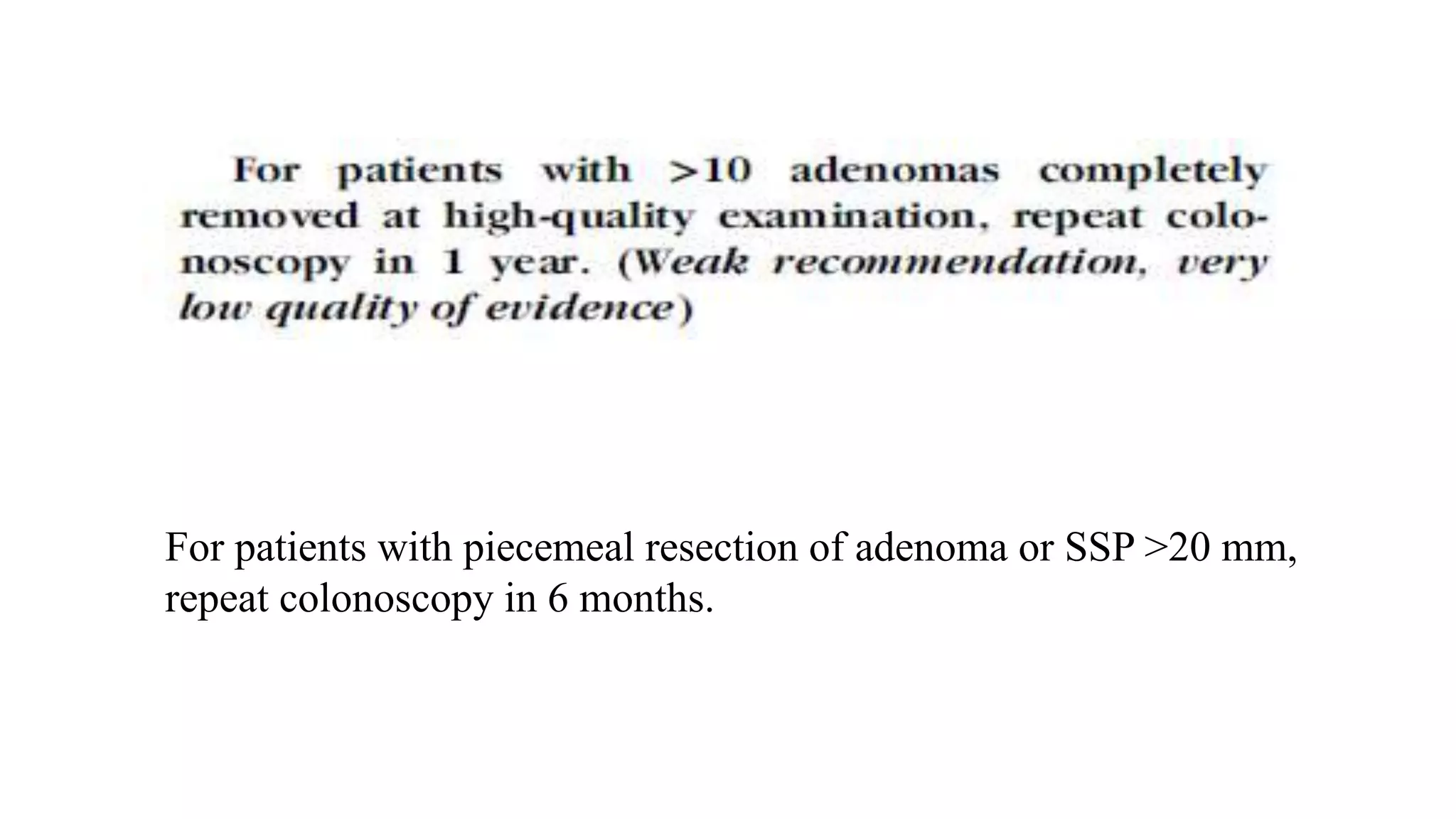
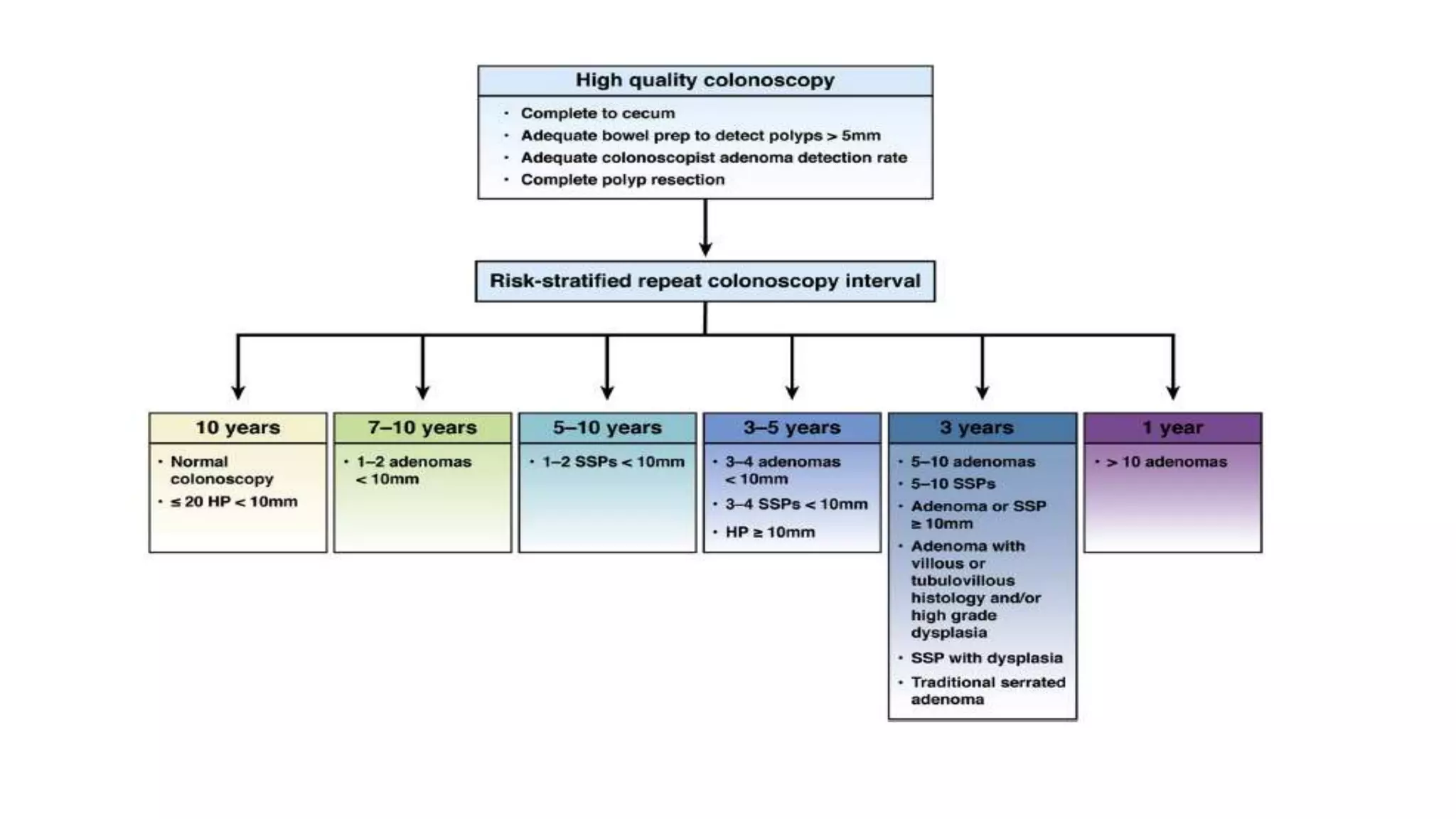
The document discusses colorectal polyps, which are discrete tissue masses that protrude into the bowel lumen, and various methods for their detection, including fecal tests, colonoscopy, and imaging techniques. It highlights the characteristics and classification of polyps, emphasizing the risk factors for malignancy and outlining management strategies such as endoscopic resection methods. Additionally, it details the histological features of different polyp types and their implications for colorectal cancer development.